Neurochemical characterization of nerve fibers in the porcine gallbladder wall under physiological conditions and after the administration of Salmonella enteritidis lipopolysaccharides (LPS)
Krystyna
Makowska
 *a,
Anita
Mikolajczyk
b,
Jaroslaw
Calka
a and
Slawomir
Gonkowski
a
*a,
Anita
Mikolajczyk
b,
Jaroslaw
Calka
a and
Slawomir
Gonkowski
a
aDepartement of Clinical Physiology, Faculty of Veterinary Medicine University of Warmia and Mazury in Olsztyn, Poland. E-mail: krystyna.makowska@uwm.edu.pl
bDepartment of Public Health, Epidemiology and Microbiology, Faculty of Medical Sciences University of Warmia and Mazury in Olsztyn, Poland
First published on 26th October 2017
Abstract
Lipopolysaccharides (LPS, bacterial endotoxin) are a component of the cellular membrane of Gram-negative bacteria, which is known as an important pathological factor. In spite of many previous studies describing multidirectional negative effects of LPS on living organisms, the knowledge concerning the influence of bacterial endotoxins on the gallbladder innervation is extremely scarce. The present study, based on the immunofluorescence technique, describes the changes in the neurochemical characterization of nerves within various parts of the porcine gallbladder (neck, body and fundus) after the administration of low doses of LPS. The obtained results show that even low doses of bacterial endotoxins affect the nerve structures within the gallbladder wall and the intensity of fluctuations in immunoreactivity to particular substances clearly depends on the part of the investigated organ. The most evident changes were observed in the case of fibers exhibiting the presence of neuropeptide Y (an increase from 7.84 ± 0.17 to 14.66 ± 0.37) in the neck, substance P (an increase from 0.88 ± 0.1 to 8.4 ± 0.3) in the body and the vesicular acetylocholine transporter in the gallbladder's fundus (an increase from 4.29 ± 0.18 to 11.01 ± 0.26). The mechanisms of the observed changes still remain unclear, but probably they are connected with the pro-inflammatory and/or neurodegenerative activity of LPS.
Introduction
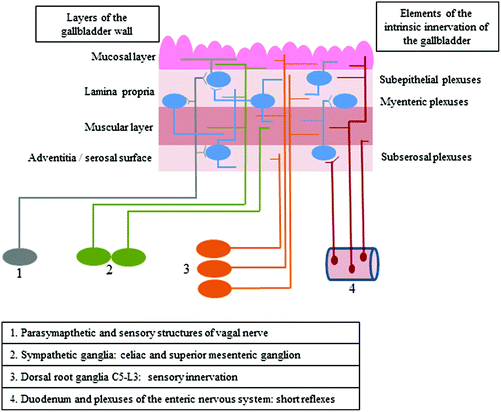
The gallbladder is supplied by both intrinsic and extrinsic innervation, which first of all takes part in regulatory processes connected with smooth muscle contractility and secretory activity of the mucosal layer (Fig. 1).1 Intrinsic innervation of the gallbladder is generally similar to the enteric nervous system (ENS) in the digestive tract and consists of neurons located in the wall of this organ, which are grouped in ganglionated plexuses.2 In contrast to the stomach and intestine, the population of neurons in the wall of the gallbladder is rather sparse, and intramural ganglia are smaller and more irregular.1 The number and exact localization of ganglionated plexuses in the wall of the gallbladder (just like in the ENS within the stomach and intestine) clearly depend on the animal species studied.1–3 In rodents, the gallbladder intramural innervation consists of two plexuses: the subserosal plexus located on the outside of the muscular layer and the subepithelial plexus near the lamina propria of the mucosa.3 In larger mammals, there are three types of plexuses in the gallbladder wall: the subserosal plexus – located in the same place as in rodents – as well as muscular and subepithelial plexuses, which are placed at different topographical levels in the mucosal lamina propria.4The extrinsic innervation of the gallbladder consists of various components. The first of them are the preganglionic cholinergic parasympathetic fibers, which are the axons of neuronal cells located in the dorsal motor nucleus of the vagal nerve.5,6 The second group of fibers supplying the wall of the gallbladder are postganglionic sympathetic nerves, being the projections of neuronal cells placed within the celiac and superior mesenteric ganglia.1,5 Moreover, sensory innervation has been described in the gallbladder wall, and sensory neurons supplying this organ are located bilaterally in both the nodose ganglia and in the dorsal root ganglia from neuromeres C5 to L3.7,8 The next groups of fibers, which have been described in the gallbladder, are the projections from enteric neurons placed in the duodenal wall and the sphincter of Oddi, which take part in short local reflex arcs between particular parts of the digestive organs without the central nervous system engagement.9,10
Both intrinsic and extrinsic neurons supplying the gallbladder use a wide range of neuronal factors which most often play the role of neuromediators and/or neuromodulators. Based on previous studies, the most important of them are (among others) acetylcholine, substance P, vasoactive intestinal polypeptide, calcitonin gene-related peptide and nitric oxide.1
Moreover, it is relatively well-known that the ENS within the stomach and intestine as well as the extrinsic innervation of the GI tract may undergo various changes in a wide range of physiological and pathological processes. These changes are of an adaptive and/or neuroprotective nature and mainly concern the neurochemical characterization of enteric neurons and may occur as a result of the growth and puberty of the digestive tract,11 changes in diet,12 intoxications,13,14 as well as intestinal and extra-intestinal diseases, including, among others, inflammatory processes, neuronal damage and Parkinson's disease.15–19
Similar changes have been observed in neuronal structures in the gallbladder wall, but in contrast to the stomach and intestine, knowledge about them is rather scarce. Previous studies have described the changes in the gallbladder nervous structures caused by maturation of the organism20 or some diseases including gallstones and cholecystitis,1,2 but many aspects connected with the reactions of gallbladder innervation on pathological factors remain unknown. Lipopolysaccharides (LPS) are one of these factors.
LPS (bacterial endotoxins) are heteropolymers composed of three parts: hydrophobic lipid A, a (inner and outer) core oligosaccharide and a highly specific O-antigen chain.21 They are components of the cellular membrane of Gram-negative bacteria and take part in the protection of bacterial cells against outside factors, such as antibiotics.22 LPS, by the activation of the immunological system and stimulation to release free radicals, may have various negative effects on living organisms.21,22 Most frequently LPS intoxication causes fever, chills, and flu-like symptoms and may result in internal organ injury, sepsis and death.23,24
As mentioned before, bacterial endotoxins, such as LPS, cause cholecystitis and disturbances in gallbladder epithelial cell functions25,26 but, to date, the influence of LPS on the innervation of the porcine gallbladder has not been studied in detail. On the other hand, pigs are more and more often used in biomedical sciences due to their relatively well-known physiological, biochemical and immunological similarities to humans.27 These similarities primarily concern the anatomy and physiology of neurons supplying the digestive system28,29 and because of this pigs seem to be an optimal (better than rodents) animal model of processes taking place in human internal organs.27,30 The results obtained during the present study may have significance, particularly because, in contrast to the majority of previous investigations, this experiment was conducted using a “low single dose”, which can simulate the asymptomatic, relatively widespread in the human population, carrier state of Salmonella spp.
The present study describes the influence of LPS on nerves localized in the gallbladder wall immunoreactive to selected active substances, such as the nervous isoform of nitric oxide synthase (nNOS – the marker of nitrergic neurons), substance P (SP), the vesicular acetylcholine transporter (VAChT, the marker of cholinergic nerves), galanin (GAL), the pituitary adenylate cyclase-activating polypeptide (PACAP), the calcitonin gene-related peptide (CGRP), neuropeptide Y (NPY) and the vasoactive intestinal polypeptide (VIP).The above mentioned substances have been selected for this study not only because of their wide distribution in the enteric nervous system but also because of their specificities. Among other activities, these substances play important roles in the maintenance of organism homeostasis after the action of bacterial endotoxins.31–38 Moreover, previous studies reported that most of them (nitric oxide, GAL, SP, VIP, NPY and CGRP) have neuroprotective functions in the enteric nervous system.14,39,40
Materials and methods
This study was performed on ten immature female pigs (aged 8 weeks, 18–20 kg body weight) of Piétrain × Duroc breed. Animals qualified for the experiment were clinically healthy. Moreover, an asymptomatic carrier state of Salmonella spp. was excluded by standard fecal analysis. During the present study, pigs were kept under standard laboratory conditions and fed with commercial feed for pigs of this age group. The study complied with all institutional and national guidelines applicable within the Republic of Poland, as per the Federal Law of 15 January 2015 on Animal Welfare for Science and Education (Dz.U.2015.0.266). The experimental protocol was approved by the Local Ethical Committee for Animal Experimentation in Olsztyn (Poland) (decision no. 73/2015 from 29th Sept 2015). The authors provided written informed consent for the study, and all experimental procedures were performed in agreement with the instructions of the above-mentioned committee.After a two-week adaptive period, the animals were randomly divided into two groups (5 pigs in each group): the control (C Group) and experimental group (LPS Group). Pigs of both groups were pre-medicated with intramuscular injection of atropine (Atropinum Sulfuricum Polfa Warszawa S.A., Poland, 0.035 mg per kg body weight – b.w.), ketamine (Bioketan, Vetoquinol Biowet Sp. z o.o., Poland & Vetoquinol S.A., France, 7.0 mg per kg b.w.) and medetomidine (Cepetor, CP-PharmaHandelsges mbH, Germany, 0.063 mg per kg b.w.). After ten minutes, the pigs of the experimental group were subjected to intravenous injection (into the marginal ear vein) with lipopolysaccharides from Salmonella enterica serotype Enteritidis (catalogue no. L7770 Sigma, Aldrich, Germany) at a dose of 5 μg per kg b.w. (in 10 ml saline solution). Such a dose has been previously described as a “low single dose”, which under experimental conditions may simulate the asymptomatic carrier state of Salmonella spp.41 The control group pigs received 10 ml of saline solution without LPS in the same way.
After seven days, the period after which the first LPS-induced changes in the nervous system have been previously described,42,43 all pigs were again pre-medicated (in the above-described manner) and after 15 min were subjected to general anesthesia using propofol (Scanofol, NORBROOK, Northern Ireland, IRL.PN, 4.5 mg per kg b.w. given intravenously) and then euthanized with sodium pentobarbital (Morbital, Biowet Puławy Sp. z o.o, Poland, 69–70 mg per kg b.w., given intravenously).
Immediately after euthanasia, gallbladders from all animals were collected. Tissues were fixed in a solution of 4% buffered paraformaldehyde (pH 7.4) for 30 min, rinsed in phosphate buffer (0.1 M, pH 7.4, at 4 °C) for three days (with a daily exchange of buffer) and put into 18% phosphate-buffered sucrose (at 4 °C). Under these conditions, tissues were stored for at least two weeks. The particular parts of gallbladders (neck, body and fundus) were then frozen at −22 °C, cut into 14 μm-thick sections using a microtome (Microm, HM 525, Walldorf, Germany), fixed on glass slides and subjected to the single-labeling immunofluorescence technique described previously by Gonkowski et al.16
Tissues were dried for 45 min and incubated (1 h) with a blocking solution (10% goat serum, 0.1% bovine serum albumin (BSA), 0.01% NaN3, Triton X-100, thimerosal in PBS). Sections of the gallbladder were then incubated (overnight; in a humidity chamber) with one primary antibody directed towards the particular substances studied, including nNOS – the marker of nitrergic neurons, SP, VAChT – the marker of cholinergic nerves, GAL, PACAP, CGRP, NPY and VIP. On the next day, the visualization of complexes “primary antibody–appropriate antigen” was performed by incubation for 1 h with species-specific secondary antisera conjugated to Alexa Fluor. All the above-mentioned procedures were carried out at room temperature and the rinsing of the slices in PBS (three times × 15 min) was performed between each stage of labelling. The precise specifications of the primary and secondary antibodies used during the present study are shown in Table 1.
| Primary antibodies | ||||
|---|---|---|---|---|
| Antigen | Code | Species | Working dilution | Supplier |
| nNOS | AB5380 | Rabbit | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2000 2000 |
Merck Millipore (Poland), Warsaw, Poland |
| SP | 8450-0505 | Rat | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000 1000 |
Bio-Rad (AbD Serotec), Kidlington, UK |
| VAChT | H-V006 | Rabbit | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2000 2000 |
Phoenix Pharmaceuticals, INC, Belmont, CA, USA |
| GAL | T-5036 | Guinea pig | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2000 2000 |
Peninsula, San Carlos, CA, USA |
| PACAP | T-5039 | Guinea pig | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000 1000 |
Chemicon International INC, Temecula, CA, USA |
| CGRP | T-5027 | Guinea pig | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1600 1600 |
Peninsula |
| NPY | NA 1115 | Rabbit | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2000 2000 |
Biomol, Hamburg, Germany |
| VIP | VA 1285 | Rabbit | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2000 2000 |
Enzo Life Sciences; Farmingdale, NY, USA |
| Secondary antibodies | ||
|---|---|---|
| Reagents | Working dilution | Supplier |
| Alexa fluor 488 donkey anti-guinea pig IgG | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000 1000 |
Invitrogen, Carlsbad, CA, USA |
| Alexa fluor 488 donkey anti-rat IgG | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000 1000 |
Invitrogen |
| Alexa fluor 546 donkey anti-rabbit IgG | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000 1000 |
Invitrogen |
Standard controls, i.e. pre-absorption of the neuropeptide antibodies with appropriate antigens (Table 2) for 18 hours at 37 °C, “omission” and “replacement” of primary antibodies by non-immune sera were performed to test the antibody and the specificity of the immunofluorescence method and these procedures completely eliminated specific stainings.
| Antigens used in pre-absorption tests | Code | Concentration | Supplier |
|---|---|---|---|
| NOS | N3033 | 1.0 μM | Sigma, St Louis, MO, USA |
| SP | S6883 | 0.7 μM | Sigma |
| VAChT | V007 | 0.6 μM | Phoenix Pharmaceuticals, INC., Belmont, CA, USA |
| GAL | G0278 | 0.5 μM | Sigma |
| PACAP | 052-02 | 0.3 μM | Phoenix Pharmaceuticals |
| CGRP | AS-20681 | 0.6 μM | AnaSpec Fremont, CA, USA |
| NPY | PEP-87135 | 0.2 μM | Dianova, Hamburg, Germany |
| VIP | V6130 | 1.0 μM | Sigma |
The evaluation of the density of nerves immunoreactive to the particular substances studied located in the neck, body and fundus of the gallbladder was performed by the counting of these fibers per microscopic observation field (0.1 mm2). The number of nerve fibers was denoted in four fragments of the particular parts of the gallbladder per pig (in five fields per section) and the obtained data were pooled and presented as mean ± SEM. A statistical analysis was conducted with an Anova-test (Statistica 9.1, StatSoft, Inc.) and the differences were considered statistically significant at p ≤ 0.05.
Results
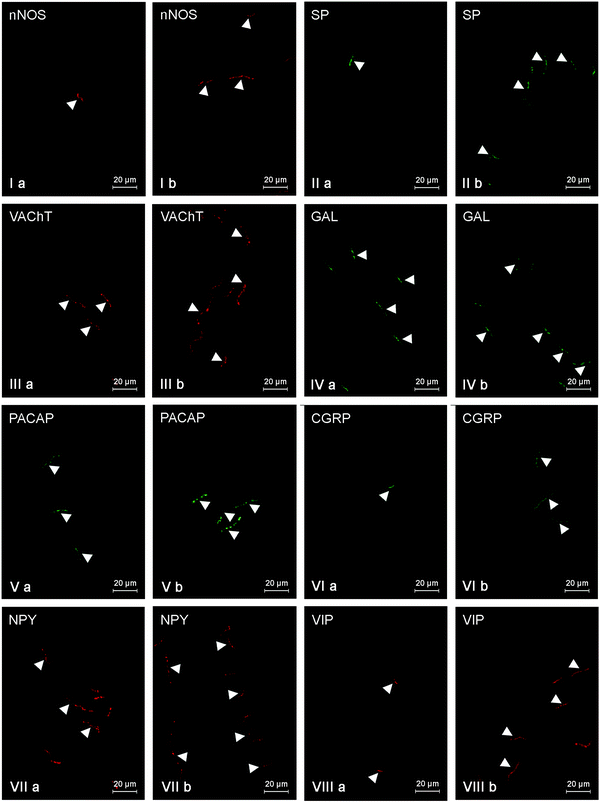
During the present experiment, nerve fibers immunoreactive to all neuronal factors studied were noted in all investigated parts of the gallbladder, both in the control animals and pigs after LPS administration. Moreover, the number of nerves immunoreactive to particular substances clearly depended on the part of the gallbladder. In addition, the influence of LPS generally caused an increase in the expression of all neuronal substances studied in the nerves of all fragments of the gallbladder, but the intensity of the observed changes was different in various parts of this organ.Under physiological conditions, most intramural nerves within the gallbladder neck (Fig. 2, Table 3) were immunoreactive to VIP (Fig. 2VIIIa) and/or VAChT (Fig. 2IIIa). The average number of such fibers per observation field amounted to 17.8 ± 0.56 and 11.9 ± 0.77, respectively. A slightly lower quantity of nerves showed the presence of GAL (9.19 ± 0.16) (Fig. 2IVa) and/or NPY (7.84 ± 0.17) (Fig. 2VIIa). Nerves immunopositive to nNOS (Fig. 2Ia) and/or PACAP (Fig. 2Va) were even rarer. Their number amounted to 3.61 ± 0.06 and 3.04 ± 0.26, respectively. In contrast, during the present study, only single SP- and/or CGRP-like immunoreactive nerves were noted in the gallbladder necks of control animals (Fig. 2IIa and VIa, Table 3). LPS administration caused an increase in the number of all investigated types of fibers within the gallbladder neck (Fig. 2, Table 3). Although most nerves within the neck of the gallbladder in animals treated with LPS were immunoreactive to VIP (21.44 ± 0.15) (Fig. 2VIIIb), the largest LPS-induced changes were noted in the case of NPY-like immunoreactive (LI) nerves (an increase from 7.84 ± 0.17 to 14.66 ± 0.37) (Fig. 2VIIb). In contrast to NPY, CGRP expression in the nerves of the gallbladder neck was subjected to the least visible changes under LPS action (Fig. 2VIb). The number of CGRP-LI nerves increased from 1.1 ± 0.03 to 2.44 ± 0.16, and the population of these fibers was less in the gallbladder neck of animals following LPS administration (Table 3).
| Active substance | C group | LPS group |
|---|---|---|
| nNOS | 3.61 ± 0.06* | 7.45 ± 0.35* |
| SP | 1.11 ± 0.03* | 5.42 ± 0.17* |
| VAChT | 11.9 ± 0.77* | 16.68 ± 0.07* |
| GAL | 9.19 ± 0.16* | 12.8 ± 0.11* |
| PACAP | 3.04 ± 0.26* | 5.77 ± 0.13* |
| CGRP | 1.1 ± 0.03* | 2.44 ± 0.16* |
| NPY | 7.84 ± 0.17* | 14.66 ± 0.37* |
| VIP | 17.8 ± 0.56* | 21.44 ± 0.15* |
Within the gallbladder body (Fig. 3, Table 4) of control animals, most of the nerves were immunoreactive to NPY (10.21 ± 0.8) and/or GAL (9.25 ± 0.05). A significantly lower density was observed in the case of VAChT-positive fibers (4.78 ± 0.29) and the number of nerves immunoreactive to other neuronal factors studied was rather scanty and the average number of them did not exceed three fibers per observation field (from 0.88 ± 0.1 in the case of SP to 2.99 ± 0.11 in the case of PACAP) (Fig. 3, Table 4). Moreover, the neurochemical characterization of nerves located in the wall of the gallbladder body under physiological conditions (Fig. 3, Table 4) was different from that observed within the gallbladder neck. The most visible differences concerned VIP- and/or VAChT-positive nerves. The number of the former amounted to 2.02 ± 0.03 (in the neck: 17.8 ± 0.56), and the latter to 4.78 ± 0.29 (in the neck: 11.9 ± 0.77). LPS administration induced an increase in the number of fibers immunopositive to all investigated neuronal factors (Fig. 3, Table 4) within the gallbladder body, which was similar to the situation observed in the neck of this organ. Under LPS action, the greatest number of fibers showed the presence of NPY (13.16 ± 0.15) (Fig. 3VIIb), and the most visible fluctuations concerned nerves immunoreactive to SP (an increase from 0.88 ± 0.1 to 8.4 ± 0.3) and/or VAChT (the increase from 4.78 ± 0.29 to 10.95 ± 0.15) (Fig. 3IIIb, Table 4).
| Active substance | C group | LPS group |
|---|---|---|
| nNOS | 1.4 ± 0.08* | 6.48 ± 0.23* |
| SP | 0.88 ± 0.1* | 8.4 ± 0.3* |
| VAChT | 4.78 ± 0.29* | 10.95 ± 0.15* |
| GAL | 9.25 ± 0.05* | 11.83 ± 0.18* |
| PACAP | 2.99 ± 0.11* | 4.65 ± 0.13* |
| CGRP | 1.57 ± 0.19* | 3.27 ± 0.05* |
| NPY | 10.21 ± 0.8* | 13.16 ± 0.15* |
| VIP | 2.02 ± 0.03* | 7.59 ± 0.06* |
The general density of fibers within the gallbladder fundus (Fig. 4, Table 5) was the least among all investigated parts of the gallbladder. In control animals, the number of fibers immunoreactive to any neurochemical factors studied did not exceed five fibers per observation field. Most of the fibers showed the presence of GAL (4.51 ± 0.24), VAChT (4.29 ± 0.18) and/or nNOS (3.39 ± 0.17) (Fig. 4, Table 5). SP- and/or NPY-LI nerves were less prolific (Fig. 4IIa and VIIa) and totaled 2.83 ± 0.13 and 2.29 ± 0.11 fibers per observation field, respectively (Table 5). The quantity of nerves immunoreactive to other substances did not exceed three fibers per observation field, and the smallest population was represented by the fibers immunoreactive to CGRP (Fig. 4VIa). Their number was 0.55 ± 0.05 fibers per observation field (Table 5). In the gallbladder fundus (just like in the other parts of the gallbladder), LPS administration led to an increase in the number of nerves immunoreactive to all substances studied (Table 5). The most evident changes were observed in the case of fibers exhibiting the presence of VAChT (an increase from 4.29 ± 0.18 to 11.01 ± 0.26) and/or NPY (an increase from 2.29 ± 0.11 to 7.05 ± 0.36) (Fig. 4IIIb and VIIb). In contrast, CGRP-LI nerves had less visible fluctuations (an increase from 0.55 ± 0.05 to 0.95 ± 0.06) (Fig. 4VIb, Table 5).
| Active substance | C group | LPS group |
|---|---|---|
| nNOS | 3.39 ± 0.17* | 7.84 ± 0.23* |
| SP | 2.83 ± 0.13* | 6.81 ± 0.29* |
| VAChT | 4.29 ± 0.18* | 11.01 ± 0.26* |
| GAL | 4.51 ± 0.24* | 7.38 ± 0.11* |
| PACAP | 1.97 ± 0.13* | 3.48 ± 0.07* |
| CGRP | 0.55 ± 0.05* | 0.95 ± 0.06* |
| NPY | 2.29 ± 0.11* | 7.05 ± 0.36* |
| VIP | 1.67 ± 0.07* | 3.65 ± 0.1* |
It should be pointed out that LPS not only changed the number of nerve fibers within the gallbladder wall, but also affected their morphology. In animals after LPS administration, nerves immunoreactive to the majority of substances studied were thick, more visible and formed bundles with varicosities, while under physiological conditions they were rather thin, delicate and short. These differences were the clearest in the case of nerves immunoreactive to VAChT, VIP and/or GAL (Fig. 2–4).
Discussion
The obtained results indicate that nerves located in the porcine gallbladder wall show wide variations in terms of their neurochemical properties. This observation generally complies with previous studies.1 To date, the neurochemical coding of nervous structures located in the gallbladder wall has been investigated in a wide range of mammals, including (among others) humans, rats, mice, guinea pigs, monkeys and possums.1,4,44,45 In spite of the interspecies differences concerning the distribution and number of neuronal structures immunoreactive to particular substances, in the majority of species the following active substances have been noted in the gallbladder innervation: acetylcholine, VIP, GAL, CGRP, SP and PACAP.1It should be pointed out that knowledge about the neurochemical coding of nerves in the porcine gallbladder wall is very scarce31,46 and the present study is the first to precisely describe the gallbladder innervation in this species. The present results only in part are in agreement with previous observations, where the immunoreactivity for VIP, NPY and GAL was the most abundant and the SP-like immunoreactivity was weak within the nervous structures of the porcine gallbladder wall.46 The present studies show that the number of nerves immunoreactive to particular substances clearly depends on the gallbladder part, for example VIP-positive nerves in the gallbladder body or NPY-like immunoreactive fibers in the gallbladder fundus, and they are not very numerous.
The wide range of substances observed in nervous structures within the gallbladder both in previous studies1,4,46 and during the present investigation is also typical of the intestinal enteric nervous system.15 These observations may suggest that neuronal factors exhibit similar functions in the intestine and gallbladder, regulating smooth muscle activity, intramural blood flow and secretory function of the mucosal layer, which has been partly confirmed by previous studies.1,47,48 On the other hand, some aspects of neuronal substance activity within the gallbladder have not been explained and differences in the chemical coding of nerves between the particular gallbladder fragments noted in the present study strongly suggest that the exact functions of neuromediators and/or neuromodulators depend on the part of the gallbladder.
One of the less known issues is the response of gallbladder innervation to the action of pathological processes. The obtained results clearly indicate that even low doses of LPS, which do not cause any clinical symptoms, may influence the neurochemical coding of nerves located in the gallbladder wall. These observations confirm previous studies in which LPS-induced changes in the neurochemical coding of nervous structures in the digestive system were described.31,32 Moreover, it is known that some neuromediators and/or neuromodulators take part in processes related to LPS activity. Namely, VIP, somatostatin and NPY are involved in the maintenance of immunological homeostasis under LPS intoxication,33–35 and GAL, NPY and SP exhibit antipyretic effects and take part in the stabilization of the body temperature under bacterial endotoxin action.36,49,50 In addition, NPY prevents hypotension during endotoxic shock.51
Thus, the changes in the immunoreactivity of nerves observed during the present study are probably connected with adaptive and/or protective processes that are a response to disturbances of homeostasis caused by the LPS action. However, the exact reasons and mechanisms of the observed fluctuations still remain not fully elucidated, all the more so because the majority of previous studies have been conducted with high doses of LPS.
First of all, they may be connected with the relatively well-known pro-inflammatory action of LPS. It is well-established that lipid A, one of the LPS components, may affect various immune cells, leading to an increase in the synthesis of pro-inflammatory factors, such as, among others, TNF-α, IL-1, IL-6 and IL-8.52,53 To date, inflammatory changes caused by LPS administration have been described in various internal organs, also including the gallbladder.25,26 Moreover, previous investigations have shown that inflammatory processes within digestive organs may affect the expression of neuronal active substances in the nervous system within the wall of the gastrointestinal tract, and these changes have a similar character to those observed during the present study.15–17 It is also known that some toxic substances (for example, mycotoxins) cause the increase in the levels of intestinal proinflammatory interleukins,40 accompanied by the changes in the immunoreactivity of enteric neurons and nerve fibers.13,14 Apart from the above-mentioned observations, suggestions concerning the relationship between the changes in the neurochemical coding of nerves and LPS-induced inflammation are more likely since the participation of a large number of neuronal factors in immunological processes has been described in previous studies. Namely, it is known that VIP can inhibit macrophage activity, resulting in a decrease in pro-inflammatory cytokine levels.54,55 Such activity of VIP has also been observed in human monocytes stimulated with LPS,54 which suggests that this neuronal factor is an effective therapeutic agent during endotoxin-induced sepsis.56 In contrast, the participation of SP in immunological processes manifests in the stimulation of NK1 receptors localized on lymphocytes and macrophages and an increase in pro-inflammatory factor levels.57 Other neuronal factors studied during the present investigation are also involved in inflammatory processes within the digestive system, as shown by the changes in their expression during various types of inflammation.15,58 On the other hand, the present study has been performed with small doses of LPS, which did not cause visible symptoms of inflammation or pain reactions (as witnessed by slight changes in the number of fibers immunoreactive to CGRP, a well-known factor involved in sensory and pain stimuli conduction). However, it is probable that subclinical inflammatory changes may also occur under the influence of low doses of LPS, but fluctuations in the immunoreactivity noted during the present study may also be connected with other mechanisms.
The second reason for the changes noted during the present study may be the neuroprotective and adaptive reactions in response to the neurodegenerative activity of LPS. This activity is usually associated with neuroinflammatory processes59 and has been described both in the central and peripheral nervous systems.60,61 LPS-induced neurodegeneration results from oxidative stress, which causes dysfunctions of mitochondria within neuronal cells.59 It should be pointed out that LPS affects neuronal tissue at relatively low doses,62 which may suggest that the changes observed during the present study also arise from the neurodegenerative actions of bacterial endotoxins. Moreover, previous studies have described the damage of enteric neurons by LPS,63 as well as the participation of some neuromediators and/or neuromodulators within the central and peripheral nervous systems in the rescue of neuronal cells affected by endotoxins.63,64 In addition, the majority of substances studied during the present study are known as important neuroprotective factors within the digestive system, whose expression usually increases during various pathological processes.15
Due to the fact that LPS has a multidirectional negative influence on living organisms,23,24 fluctuations observed during the present study may result from other mechanisms. Namely, they can be connected with LPS-induced changes in synaptic transmission65 and/or disturbances of sensory stimuli conduction.66 However, the latter of these mechanisms is rather unlikely, because changes in the number of nerves immunoreactive to CGRP (an important sensory factor) were very slight. Admittedly, fluctuations in the population of fibers immunopositive to SP – the second (along with CGRP) substance involved in sensory and pain stimuli conduction – were clearly visible, but they could have been connected with the immunological and/or neuroprotective activity of substance P. In addition, the changes observed during the present study may have resulted from adaptive processes arising from the influences of LPS described in the previous studies on the gallbladder mucosal cells26 and/or the blood flow in the digestive system.67 It should be pointed out that the direct causes of fluctuations in the immunoreactivity of nerves in the gallbladder wall are not fully explained. They may arise from disturbances at various stages of peptide synthesis, including transcription, translation, and post-translational modifications, as well as from changes in axonal transport.
Conclusions
In conclusion, this study indicates that nervous structures in the porcine gallbladder wall are characterized by wide variations in terms of neurochemical coding. In addition, the significant differences between the particular gallbladder fragments suggest that exact roles of neuronal factors depend on the part of the investigated organ. Moreover, the obtained results clearly show that even low doses of LPS, which do not cause any clinical symptoms, may affect the neurochemical coding of intramural nerves within the porcine gallbladder wall. The observed changes are probably connected with subclinical inflammatory processes or the neurodegenerative activity of LPS, but the determination of the exact mechanisms responsible for them requires further study.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
Funding for the study was provided from a statute grant of the Faculty of Medical Sciences of the University of Warmia and Mazury, no. 25.610.001-300.References
- O. B. Balemba, M. J. Salter and G. M. Mawe, Anat. Rec., Part A, 2004, 280, 836–847 CrossRef PubMed.
- V. Villanacci, R. Del Sordo, M. Salemme, M. Cadei, A. Sidoni and G. Bassotti, Dig. Liver Dis., 2016, 48, 792–795 CrossRef PubMed.
- G. M. Mawe, in Neurogastroenterology: from the basics to the clinics, ed. H. J. Krammer and M. V. Singer, Kluwer Academic Publishers and Falk Foundation, New York, 2000 Search PubMed.
- A. C. Meedeniya, A. C. Schloithe, J. Toouli and G. T. Saccone, Neurogastroenterol. Motil., 2003, 15, 383–392 CrossRef CAS PubMed.
- G. M. Maweand and M. D. Gershon, J. Comp. Neurol., 1989, 283, 374–390 CrossRef PubMed.
- A. J. Li, J. Z. Liu and C. Y. Liu, Chin. J. Physiol., 2002, 45, 19–24 Search PubMed.
- K. Iwahashi, R. Matsuda and K. Tsunekawa, J. Auton. Nerv. Syst., 1991, 32, 145–151 CrossRef CAS PubMed.
- G. A. Iwamoto, T. G. Waldrop, J. C. Longhurstand and G. A. Ordway, Exp. Neurol., 1984, 84, 709–714 CrossRef CAS PubMed.
- R. T. Padbury, J. B. Furness, R. A. Baker, J. Toouli and J. P. Messenger, Gastroenterology, 1993, 104, 130–136 CrossRef CAS.
- J. H. Seo, S. S. Cho, I. S. Lee and H. S. Lee, Arch. Histol. Cytol., 2002, 65, 317–321 CrossRef PubMed.
- S. Hetz, A. Acikgoez, C. Moll, H. G. Jahnke, A. A. Robitzki, R. Metzger and M. Metzger, Front. Aging Neurosci., 2014, 6, 276 Search PubMed.
- P. P. Bertrand, K. E. Polglaze, H. Chen, S. L. Sandow, A. Walduck, T. A. Jenkins, R. L. Bertrand, A. E. Lomax and L. Liu, Adv. Exp. Med. Biol., 2016, 891, 201–211 CrossRef PubMed.
- K. Makowska, S. Gonkowski, L. Zielonka, M. Dabrowski and J. Calka, Neurotoxic. Res., 2017, 31, 136–147 CrossRef CAS PubMed.
- K. Makowska, K. Obremski, L. Zielonka and S. Gonkowski, Toxins (Basel), 2017, 9 CrossRef PubMed , pii: E98.
- V. Vasina, G. Barbara, L. Talamonti, V. Stanghellini, R. Corinaldesi, M. Tonini, F. De Ponti and R. De Giorgio, Auton. Neurosci., 2006, 126–127, 264–272 CrossRef CAS PubMed.
- S. Gonkowski, B. Kaminska, P. Burlinski, A. Kroll and J. Calka, Przegl. Gastroenterol., 2009, 4, 147–151 CrossRef.
- S. Gonkowski, P. Burlinski, P. Szwajca and J. Calka, Bull. Vet. Inst., 2012, 56, 199–203 Search PubMed.
- J. Wojtkiewicz, M. Równiak, R. Crayton, S. Gonkowski, A. Robak, M. Zalecki, M. Majewski and L. Klimaschewski, J. Mol. Neurosci., 2013, 51, 99–108 CrossRef CAS PubMed.
- C. Pellegrini, R. Colucci, L. Antonioli, E. Barocelli, V. Ballabeni, N. Bernardini, C. Blandizzi, W. J. de Jonge and M. Fornai, Neurogastroenterol. Motil., 2016, 28, 1781–1791 CrossRef CAS PubMed.
- G. P. Siou, A. Belai and G. Burnstock, Cell Tissue Res., 1994, 276, 61–68 CrossRef CAS PubMed.
- A. Steimle, I. B. Autenrieth and J. S. Frick, Int. J. Med. Microbiol., 2016, 306, 290–301 CrossRef CAS PubMed.
- C. Whitfield and M. S. Trent, Annu. Rev. Biochem., 2014, 83, 99–128 CrossRef CAS PubMed.
- Z. Mohammadi, J. Calif. Dent. Assoc., 2011, 39, 152–155 Search PubMed.
- R. F. Maldonado, I. Sá-Correia and M. A. Valvano, FEMS Microbiol. Rev., 2016, 40, 480–493 CrossRef CAS PubMed.
- D. L. Kaminski, W. K. Feinstein and Y. G. Deshpande, Prostaglandins, 1994, 47, 233–245 CrossRef CAS.
- D. L. Kaminski, G. Amir, Y. G. Deshpande, D. Beck and A. P. Li, Prostaglandins, 1994, 47, 319–330 CrossRef CAS.
- N. Verma, A. W. Rettenmeier and S. Schmitz-Spanke, Proteomics, 2011, 11, 776–793 CrossRef CAS PubMed.
- D. R. Brown and J. P. Timmermans, Neurogastroenterol. Motil., 2004, 16, 50–54 CrossRef PubMed.
- J. C. Litten-Brown, A. M. Corson and L. Clarke, Animal, 2010, 4, 899–920 CrossRef CAS PubMed.
- J. K. Patterson, X. G. Lei and D. D. Miller, Exp. Biol. Med., 2008, 233, 651–664 CrossRef CAS PubMed.
- A. Mikolajczyk and K. Makowska, Folia Morphol., 2017 DOI:10.5603/FM.a2017.0036.
- U. Voss and E. Ekblad, PLoS One, 2014, 9, e114044 Search PubMed.
- F. ter Veld, B. Rose, R. Mussmann, S. Martin, C. Herder and K. Kempf, J. Endocrinol. Invest., 2009, 32, 123–129 CrossRef CAS PubMed.
- R. Ferreira, T. Santos, M. Viegas, L. Cortes, L. Bernardino, O. V. Vieira and J. O. Malva, J. Neuroinflammation, 2011, 8, 169 CrossRef CAS PubMed.
- L. Fraccaroli, E. Grasso, V. Hauk, M. Cortelezzi, C. Pérez Leirós and R. Ramhorst, NeuroImmunoModulation, 2014, 21, 21–30 CrossRef CAS PubMed.
- V. Lyudyno, I. N. Krasnova, M. P. Smirnova and V. M. Klimenko, Bull. Exp. Biol. Med., 2001, 131, 60–63 CrossRef CAS PubMed.
- B. Tunctan, E. Ozveren, B. Korkmaz, C. K. Buharalioglu, L. Tamer, U. Degirmenci and U. Atik, Pharmacol. Res., 2006, 53, 177–192 CrossRef CAS PubMed.
- K. Elekes, K. Sandor, A. Moricz, L. Kereskai, A. Kemeny, E. Szoke, A. Perkecz, D. Reglodi, H. Hashimoto, E. Pinter, J. Szolcsanyi and Z. Helyes, Peptides, 2011, 32, 1439–1446 CrossRef CAS PubMed.
- J. Wojtkiewicz, M. Równiak, R. Crayton, M. Barczewska, M. Bladowski, A. Robak, Z. Pidsudko and M. Majewski, J. Mol. Neurosci., 2012, 46, 450–458 CrossRef CAS PubMed.
- K. Obremski, S. Gonkowski and P. Wojtacha, Pol. J. Vet. Sci., 2015, 18, 357–365 CAS.
- D. M. Webel, B. N. Finck, D. H. Baker and R. W. Johnson, J. Anim. Sci., 1997, 75, 1514–1520 CrossRef CAS PubMed.
- L. Qin, X. Wu, M. L. Block, Y. Liu, G. R. Breese, I. S. Hong, D. J. Knapp and F. T. Crews, Glia, 2007, 55, 453–462 CrossRef PubMed.
- V. Rivera-Aguilar, E. Querejeta, R. A. Jarillo-Luna, H. Reyna-Garfias, D. Ponce-Franco, A. Milliar-Garcia, A. R. Quiñones-Cárdenas, J. Pacheco-Yepez and R. Campos-Rodríguez, Immunol. Lett., 2008, 120, 20–28 CrossRef CAS PubMed.
- G. M. Mawe and L. M. Ellis, Anat. Rec., 2001, 262, 101–109 CrossRef CAS PubMed.
- E. K. Talmage, W. A. Pouliot, M. Schemann and G. M. Mawe, Cell Tissue Res., 1996, 284, 289–302 CrossRef CAS PubMed.
- J. Sand, H. Tainio and I. Nordbsck, Dig. Dis Sci., 1993, 38, 694–700 CrossRef CAS PubMed.
- C. Aydin, I. Bagcivan, S. Yildirim, A. Koyuncu, O. Topcu, S. Soylu and H. Ozer, Eur. Surg. Res., 2009, 42, 189–194 CrossRef CAS PubMed.
- H. C. McKirdy, Am. J. Physiol.: Gastrointest. Liver Physiol., 2008, 295, G209 CrossRef CAS PubMed.
- M. Felies, S. von Hörsten, R. Pabst and H. Nave, J. Physiol., 2004, 561, 245–252 CrossRef CAS PubMed.
- H. O. Brito, F. L. Barbosa, R. C. Reis, D. Fraga, B. S. Borges, C. R. Franco and A. R. Zampronio, J. Neuroimmunol., 2016, 293, 1–7 CrossRef CAS PubMed.
- G. J. Hauser, E. K. Dayao and Z. Zukowska-Grojec, Life Sci., 1995, 57, 235–244 CrossRef CAS PubMed.
- S. Coquenlorge, E. Duchalais, J. Chevalier, F. Cossais, M. Rolli-Derkinderen and M. Neunlist, J. Neuroinflammation, 2014, 11, 202 CrossRef PubMed.
- A. Gnauck, R. G. Lentle and M. C. Kruger, Int. Rev. Immunol., 2016, 35, 189–218 CrossRef CAS PubMed.
- B. Askar, H. Ibrahim, P. Barrow and N. Foster, Peptides, 2015, 71, 188–195 CrossRef CAS PubMed.
- P. M. Higyno, P. F. Mendes, M. B. Miranda, D. E. Pereira, A. P. Mota, O. Nogueira Kde, I. S. Caldas, S. A. Moura and C. A. Menezes, Exp. Parasitol., 2015, 159, 72–78 CrossRef CAS PubMed.
- H. Ibrahim, P. Barrow and N. Foster, Endocr., Metab. Immune Disord.: Drug Targets, 2012, 12, 308–315 CrossRef CAS.
- Y. Shimizu, H. Matsuyama, T. Shiina, T. Takewaki and J. B. Furness, Cell. Mol. Life Sci., 2008, 65, 295–311 CrossRef CAS PubMed.
- S. Gonkowski, P. Burlinski and J. Calka, Acta Vet. – Beograd., 2009, 59, 321–330 CrossRef.
- M. S. Khan, T. Ali, M. W. Kim, M. H. Jo, M. G. Jo, H. Badshah and M. O. Kim, Neurochem. Int., 2016, 100, 1–10 CrossRef CAS PubMed.
- A. Miura, H. Hino, K. Uchida, S. Inoue and T. Tateda, J. Anesth., 2016, 30, 961–969 CrossRef PubMed.
- R. Hunter, U. Ojha, S. Bhurtel, G. Bing and D. Y. Choi, Neurosci. Res., 2017, 114, 62–69 CrossRef CAS PubMed.
- C. L. Murray, D. T. Skelly and C. Cunningham, J. Neuroinflammation, 2011, 8, 50 CrossRef CAS PubMed.
- M. B. Arciszewski, E. Sand and E. Ekblad, Regul. Pept., 2008, 146, 218–223 CrossRef CAS PubMed.
- L. Bai, X. Zhang, X. Li, N. Liu, F. Lou, H. Ma, X. Luo and Y. Ren, Mol. Med. Rep., 2015, 12, 1002–1008 CrossRef CAS PubMed.
- F. Garcia-Oscos, D. Peña, M. Housini, D. Cheng, D. Lopez, R. Cuevas-Olguin, N. Saderi, R. Salgado Delgado, L. Galindo Charles, H. Salgado Burgos, S. Rose-John, G. Flores, M. P. Kilgard and M. Atzori, J. Neurosci. Res., 2015, 93, 859–865 CrossRef CAS PubMed.
- S. Benson, J. Kattoor, A. Wegner, F. Hammes, D. Reidick, J. S. Grigoleit, H. Engler, R. Oberbeck, M. Schedlowski and S. Elsenbruch, Pain, 2012, 153, 794–799 CrossRef CAS PubMed.
- A. Li, L. Dong, M. L. Duan, K. Sun, Y. Y. Liu, M. X. Wang, J. N. Deng, J. Y. Fan, B. E. Wang and J. Y. Han, Microcirculation, 2013, 20, 617–628 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2018 |