A versatile iron(ii)-based colorimetric sensor for the vapor-phase detection of alcohols and toxic gases†
Abstract
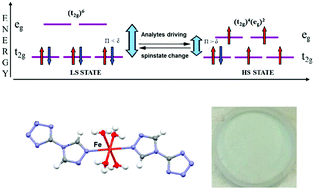
A mononuclear iron(II) neutral complex (1) was screened for colorimetric sensing abilities for a wide spectrum of vapor-phase analytes including toxic gases. Although 1 possesses a single sensing unit without any other cross-reactive elements, it can capture information about analyte molecules in a distributed fashion that is encoded sufficiently to allow discrimination from other closely related chemical structures. Clear color differentiation among four different toxic industrial chemicals (TICs) and alcohols was demonstrated. Different TICs and alcohols were readily identified using a standard chemometric approach (hierarchical clustering analysis), with no misclassifications over 18 trials. 57Fe Mössbauer spectroscopy was applied to investigate the driving spin state change of the analytes in this Fe-azole sensor material. This approach opens up perspectives for the further development of iron materials as potential optical sensor arrays through colorimetric techniques.


 Please wait while we load your content...
Please wait while we load your content...