Porous organic/inorganic hybrid one-dimensional photonic crystals for rapid visual detection of organic solvents†
Abstract
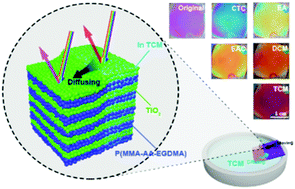
Stimuli-responsive photonic crystals (PCs) recently have bloomed into a fast-growing research area arousing wide scientific enthusiasm. Here, we propose a kind of highly sensitive and simple liquid organic solvent-responsive one-dimensional photonic crystal (1DPC) sensor derived from porous poly(methyl methacrylate–acrylic acid–ethyleneglycol dimethacrylate)/titania (P(MMA–AA–EGDMA)/TiO2) with structural colors. These organic/inorganic hybrid 1DPCs with textural porosity are fabricated through layer-by-layer assembly based on spin-coating of a microemulsion of polymer nanoparticles and a suspension of TiO2 nanoparticles on silicon substrates. The larger refractive index contrast allows the desired reflectivity to be achieved with a few layers of the 1DPCs. Tunable optical properties of the 1DPC sensors are achieved by modulating the nanoparticle concentration, rotational speed, spin-coating times and stack number. The solvent tunability of the sensors is due to the dependence of the layer refractive index and thickness of the 1DPCs on solvents. Notably, owing to the porosity generated from the nanoparticle-based structure and the high sensitivity of the crosslinked polymer nanoparticles to organic solvents, the obtained 1DPCs present a rapid (within 2 s), obvious and distinguishable color change when immersed in different organic solvents, and the visual detection process shows good reversibility. In addition, the 1DPC sensors also show different responses to various concentrations of organic solvents in water, such as ethanol, methanol and acetone. The porous organic/inorganic hybrid 1DPCs offer high potential for the development of economical and visually detective solvent sensors with high performance.
- This article is part of the themed collection: Photonics


 Please wait while we load your content...
Please wait while we load your content...