Open Access Article
Open Access ArticleAmorphous polyphosphate, a smart bioinspired nano-/bio-material for bone and cartilage regeneration: towards a new paradigm in tissue engineering
Xiaohong
Wang
 *,
Heinz C.
Schröder
and
Werner E. G.
Müller
*,
Heinz C.
Schröder
and
Werner E. G.
Müller
 *
*
ERC Advanced Investigator Grant Research Group at the Institute for Physiological Chemistry, University Medical Center of the Johannes Gutenberg University, Mainz, Duesbergweg 6, 55128 Mainz, Germany. E-mail: wmueller@uni-mainz.de; wang013@uni-mainz.de; Fax: +49-6131-39-25243; Tel: +49-6131-39-25910
First published on 12th April 2018
Abstract
Recent developments in the field of biomaterials for tissue engineering open up new opportunities for regenerative therapy and prevention of progression of osteo-articular damage/impairment. A key advancement was the discovery of the regenerative activity of a group of physiologically occurring high-energy polymers, inorganic polyphosphates (polyP). These bio-polymers, in suitable bioinspired formulations, turned out to be capable of inducing proliferation and differentiation of mesenchymal stem cells into osteogenic or chondrogenic lineages through differential gene expression (morphogenetic activity). Unprecedented is the property of these biopolymers to deliver high-energy phosphate in the extracellular space to promote anabolic processes including extracellular matrix synthesis in bradytrophic tissues such as cartilage and mineralized bone. This review summarizes the biological effects of these unique bio-polymers, not yet met by other biomaterials and depending on their specific formulation as smart amorphous nanoparticles/microparticles with different counterions. In addition, polyP in combination with other, hydrogel-forming polymers provides the basis for the fabrication of hardenable bio-inks applicable in additive manufacturing/3D printing and 3D cell bioprinting of regeneratively active patient-specific osteo-articular implants. The future prospects of this innovative technology are discussed.
1. Introduction
Osteo-articular impairments, especially of the joints, are major health problems in the aging society of the 21th century and pose complex challenges in research within the field of regenerative medicine. In 2010, about 10 million individuals over age 50 were estimated to suffer from osteoporosis in the United States, and another 43 million to have low bone mass; these values will increase by 2020 to 12 million cases of osteoporosis and over 52 million cases of low bone mass.1 Focusing only on knee replacement surgery the number of surgical interventions by 2030 is expected to jump nearly to 3.5 million surgeries per year.2,3 Besides autologous bone grafts, the bone implants used today are made of bioinert titanium or ceramics and with an increasing percentage of biomaterials. Presently biologization of inorganic implant materials with biomaterials is being successfully developed.4In the coming years it can be expected that new generations of bone implant materials will be developed, which are based on the biological properties and features of any multicellular organism, the “regeneration”. Therefore, new biomaterials, in particular “smart biomaterials” are needed that “actively participate in the formation of functional tissue”.5 The present day research activities in the field of regenerative medicine in orthopedics are focused on therapy (mainly cell therapy, or acellular) and their applications (orthopedic and musculoskeletal disorders, as well as oncology).6 In addition, the future implants will become personalized which refers to the application of the respective implant for an individual patient.
The underlying processes that lead to the development and formation of bones and joints are complex and comprise multi-component systems, such as the musculoskeletal system built of interfaces between different tissues, soft and hard. The soft tissue comprises osteotendious junction(s) (bone–ligament/tendon) and osteochondral junction(s) (bone–cartilage) and the hard tissue consists of mineralized calcium phosphate (hydroxyapatite). Also the composition of the hard tissue is highly complex since gradual interfaces of sequentially increasing degrees of mineralization of bone within the bone matrix exist. Based on these anatomical/morphological findings biological/biochemical deciphering of the organic, inorganic/organic and inorganic pathways turns out to be challenging.
A thorough understanding of the processes of regeneration is crucial for the successful transformation of the scientific principles and findings to applied research, with respect to biomedical applications. All multicellular animals (metazoans) comprise immortal stem cells that have the potency to generate an unlimited number of progenitor cells.7 Cells can self-renew and/or produce differentiated cells basically through the following two ways; first, by proliferation of stem cells producing differentiated cells, by de- and subsequently by re-differentiation or by trans-differentiation, and second, by proliferation of adult-derived pluripotent progenitor cells producing differentiated progeny cells or by different lineage-restricted progenitor cells each of them producing different differentiated cells.8 The application of these findings has advanced the field of regenerative biology which aims to restore in adult organisms the form and function of damaged tissues. At present three restrictions/drawbacks are formulated.8 First, the elucidation of the mechanistic aspects of embryonic development is still in the stage of basic research; second, the results gathered from adult tissue turnover and replacement in evolutionary distant vertebrate species only slowly contribute to a further advancement of the deciphering of the mechanism of regeneration in later evolved mammals; and third, despite the huge scientific and public interest in the importance of stem cells in regenerative medicine, their clinical application is still limited.9–11
It is imperative to mention that bone and cartilage, and here especially osteo-articular tissue that can be affected by bone and joint disorders, comprise only a small percentage of cells. Bone, as a hard calcified tissue, comprises both inorganic and organic extracellular components that form the structural scaffold. In contrast, the semi-rigid cartilage is built, in addition to water (75%), exclusively of organic macromolecules like aggrecan and proteoglycans (10%); among them collagen fibers are dominant (20%).12 The dominant cellular constituents of bone tissue are the osteoblasts (forming the bone material), osteocytes (present within the mature bone tissue and living as long as the organism itself), osteoclasts (degrading bone) and lining cells (involved in coupling bone resorption to bone formation). In cartilage tissue chondroblasts (perichondrial cells which develop to chondrocytes) and chondrocytes (producing and maintaining the cartilaginous matrix) are dominant.12 Both cell lineages, osteoblasts and chondrocytes, originate from osteochondroprogenitor cells which arise from mesenchymal stem cells [MSC] in the bone marrow. They differentiate into osteoblasts or chondrocytes, depending on the signaling molecules they are exposed to. Recent studies have shown the inherent potential of MSCs, the healing capability, to differentiate either into bone, cartilage or fat tissue, by improving angiogenesis and preventing fibrosis.13 Even more, they exhibit an anabolic role in tissue repair and tissue regeneration, especially during small bone-healing problems and early stages of local bone defects, but also for osteo-articular diseases.14
Preclinical and clinical studies support the application of MSCs in the therapy of adult osteo-articular disorders like as in nonunion of smaller fractures, of bone cysts and during osteonecrosis.15 Encouraging are also studies that report the beneficial use of bone marrow MSCs, bone marrow aspirates and platelet lysates, in both chondral and meniscus repair in two limited case studies.16 Persuading was the report which summarized that after MSC therapy only 7% of the patients requiring total knee replacement had to receive the implant.17 The major hurdles to be overcome prior to applying the MSC in a safe way for therapeutic use in tissue regeneration are, first, the lack of cost-effective bioprocesses for the upscaling of the cells and, second, patient-oriented protocols for the cell culture systems.18 With respect to the latter issue, the major difficulty is the growth restriction of cells at high cell densities in the culture. Generally, the cell viability is better maintained, and the cell–cell signaling is strongly promoted in high-density cultures, like 7.5 × 106 to 10 × 106 cells per ml. However, in those cultures the restricted nutrient and energy supply to the cells is a serious limitation.19 The MSCs are supplied to the patients via the intra-articular or the intravenous route.20,21 Furthermore, the MSC engraftment techniques, used today, are still prone to problems in optimization; therefore, intra-femoral injection has been applied and found to have the potency to allow efficient regeneration of MSCs.22,23 In these approaches the cells are suspended in medium (e.g., Dulbecco's Modified Eagle's medium) or phosphate-buffered saline prior to injection. It might be stressed here that it is still under debate whether articular cartilage has an own endogenous stem/progenitor cell population, since the in vivo data with those cells show only marginal healing capacities.24
2. Bone mineral deposition
Bone is a biomineral. The term “biomineralization” was coined by H. K. Erben during the first meeting on biomineralization, held in Mainz.25 This process should explain the biochemical and colloidochemical secretion steps during the formation of the inorganic deposits onto the organic template. Later on, this concept was substantially advanced.26–28 The crucial issues of the active participation both of enzymes and of the energy balance have not been addressed.In the last few years a change in the understanding of the processes involved in biomineralization has occurred. It has been disclosed that not only the metabolic processes that lead to the synthesis of organic metabolites but also the processes that result in inorganic depositions in the body follow the same rules. This means that the exergonic reactions during biomineral formation proceeding in the living system can also be controlled by the level of activation energy which is lowered by the participation of enzymes.
2.1 Enzymes and their pathways: biochemical processes resulting in osteo-articular tissue formation
It has been highlighted that the formation of the mineral phase is not a passive deposition of the minerals directly from a saturated solution onto an organic template, but a highly controlled process starting from the unstable amorphous phases and processed, again onto organic template(s), into crystalline mature product(s). Based on these paradigms it has been successfully attempted to explain mammalian bone formation as a highly complex interaction between organic and inorganic components/layers and is described by up to 7 hierarchical levels of organization.29 Skeletogenesis is genetically controlled and involves a series of specific transcription factors and growth hormones/cytokines (see: ref. 30 and 31) and contains two distinct tissues, cartilage and bone, and three specific cell types, chondrocytes in cartilage and osteoblasts and osteoclasts in bone. It is interesting to note that the osteoblasts, the chondroblasts and also the tenocytes, forming the tendons, develop (most likely) from MSCs (see: ref. 32 and 33).Basically the process of bone formation can proceed via two different mechanisms. In most skeletal structures, it occurs through endochondral ossification, a process during which mesenchymal cells accumulate and differentiate into chondrocytes to build the cartilage template for future bone. Subsequently, this organic template is replaced in parallel with the vascular invasion by bone cells which initiate mineral deposition (endochondral ossification). In a few other skeletal elements, like a part of the clavicle and of the skull, the mesenchymal cells directly differentiate into bone-forming osteoblasts, a process called intramembranous ossification. The latter process is seen also during natural healing of bone fractures (Fig. 1).
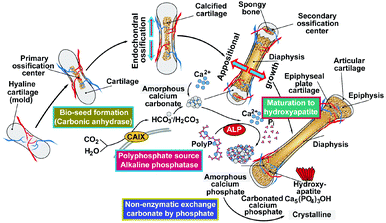 | ||
| Fig. 1 Steps during bone formation. During endochondral ossification the hyaline cartilage acts as a template mold for the initial mineralization, most likely of calcium carbonate. In parallel an ingrowth of blood vessels occurs, followed by the formation of the primary ossification centers in the diaphysis. Later, spongy bone is also formed in the epiphyses at the secondary ossification centers, with two regions of the hyaline cartilage remaining on the surface of the epiphysis (articular cartilage) and the epiphyseal plate (growth region) between the epiphysis and the diaphysis. Appositional growth of the bone proceeds in the absence of a cartilage template. Mineral deposition involves two enzymes; first, carbonic anhydrase and second, alkaline phosphatase [ALP]. This process can be subdivided into four stages. Phase I: bio-seed deposition catalyzed by carbonic anhydrase; it is postulated that the product is amorphous calcium carbonate. Phase II: hydrolytic cleavage of polyP by ALP allowing, phase III: the released phosphate units to be transferred non-enzymatically to the calcium carbonate under conversion to amorphous calcium phosphate. Phase IV: maturation of the calcium phosphate to crystalline hydroxyapatite. (Partially from ref. 36 [with permission].) | ||
During endochondral ossification bone develops by replacing hyaline cartilage. However, the cartilage is not transformed into bone, but serves as a template for new bone formation. Hence, endochondral ossification takes much longer than intramembranous ossification. Vertebrate bone, as a biomineral, is composed of a mineral phase (Ca-deposits; 60 to 70% w/w) and an organic matrix (mainly collagen; ≈20 to 30% w/w) and 10% of water (reviewed in ref. 34–37). This mineralic scaffold in the bone is tunely regulated in an interplay between the bone-forming cells (osteoblasts) and the bone-resorbing cells (osteoclasts) which are organized around the complex organic extracellular (fibrillar) mesh of macromolecules forming a three dimensional porous scaffold. Again in the extracellular space the collagen matrix undergoes mineralization primarily around collagen fibrils that function as the basic building blocks of the bone. In addition to those fibrillar proteins, non-collagenous proteins act as a second framework in a regulatory way during the mineralization process. Looking at the evolutionary origin of the metazoan skeleton the hard mineral fraction consisting mainly of calcium carbonate was used over millions of years (500 MYA) to build the skeleton of marine animals;36 later, this mineral was replaced by calcium phosphate [Ca-P], finally in the form of calcium hydroxyapatite [HA] (Ca5[PO4]3[OH]) (reviewed in ref. 37 and 38). However, also in vertebrates bone mineral contains, in addition to Ca-P, around 5% w/w carbonate, such as carbonate–fluorapatite (francolite).39 The carbonate units were proposed to exist in the apatite crystal lattices as CO32− ions, most likely by substituting for PO43− and/or OH− ions (reviewed in ref. 40). Topographically, carbonate has also been localized in vivo within the crystalline calcium carbonate (CaCO3) within the medullary bone.41 Within the bone CO2 compartment fractions of 30% bicarbonate (HCO3−) and 70% carbonate (CO32−) have been determined.42 Furthermore, in humans/mammals, besides the Ca-phosphate-based skeletal elements, biomineral deposits exist that are predominantly formed of CaCO3, e.g., the biomineralized otoliths in the vestibular labyrinth of the ear.43,44 Interestingly enough the different phases of carbonates in the otoliths are stabilized by organic axes, like the otolin or the otoconins.45
The basic principle in biochemistry, and this holds true also for the biomineralization process in a given organ, is that the formation of (almost) any stable/covalent linkage identified in living systems is catalyzed by enzymes. This is a general rule for organo-chemical reactions.46 However, until the turn of this millennium no enzyme was identified that accelerates the reaction velocity of inorganic reactants (substrates to product). In the process of the disclosure of the earliest body plan of a metazoan organism47 we have been stuck with the enzymatic basis for the skeleton formation of the phylogenetically oldest animals, the siliceous sponges. During the elucidation of the enzyme cathepsin in the sponge Geodia cydonium,48 we, together with the group of Morse,49–51 disclosed an enzyme, existing in the axial canal of the sponge Suberites domuncula, termed silicatein, that crucially participates in the polycondensation of silicic acid to bio-silica, the inorganic skeletal framework of the skeletal elements of the sponges the spicules. Applying an extraction procedure, omitting hydrofluoric acid that has been initially used, we discovered that silicatein is a genuine enzyme with a Michaelis–Menten constant [Km] and the maximal reaction velocity (Vmax);52 the enzyme has been patented.53 The parameters of silicatein have been worked out with a temperature optimum of 20–25 °C; the temperature coefficient (Q10) decreases by 2.5-fold above 25 °C and decreases by 2.9-fold below 25 °C. Using a bis(p-aminophenoxy)-dimethylsilane substrate the Km was determined to be 22.7 μM. The turnover value for silicatein in the silica esterase assay (molecules converted per enzyme molecule per second) was 5.2; in comparison the corresponding value for the human cathepsin L enzyme was determined to be 20 (reviewed in ref. 54 and 55). These data, listed here, convincingly show that the silicatein-mediated bio-silica formation is enzyme driven.
Bone formation can be subdivided into four phases, as sketched in Fig. 1 (see legend) and described in the following.
2.2 Bio-seed formation: calcium carbonate–carbonic anhydrase
Based on the published data suggesting that calcium carbonate exists within the HA-based bone scaffold we searched for an enzyme that might be involved in the initial mineral deposition onto the bone-like SaOS-2 cells. Inspired by our finding that calcium carbonate is formed, again in sponges in the Calcarea group, we disclosed that the prime candidate is carbonic anhydrase.56 In these cells mineralization is strongly upregulated by bicarbonate and the enzyme carbonic anhydrase becomes upregulated.57 In a detailed study the enzyme has been pinpointed with the membrane-associated carbonic anhydrase IX58 and a hypothesis was coined that the enzymatic product of this carbonic anhydrase is amorphous calcium carbonate around which calcium phosphate becomes deposited.59 Next we had to elucidate by which mechanism amorphous calcium carbonate, acting as a bio-seed, becomes transformed into amorphous calcium phosphate.2.3 Non-enzymatic exchange of carbonate by phosphate in amorphous calcium carbonate
Both Ca-phosphate formation60 and Ca-carbonate deposition61 are exergonic processes. However, in contrast to amorphous calcium carbonate formation, which is enzymatically driven, the exchange of carbonate by phosphate in amorphous calcium carbonate occurs even under physiological conditions without the participation of an enzyme;62 the reaction is an exergonic one. If amorphous calcium carbonate is exposed to phosphate buffer a transfer of phosphate to amorphous calcium carbonate proceeds resulting in the formation of amorphous calcium phosphate. In turn, the latter material sequentially undergoes a phase transition to HA.622.4 Origin of the phosphate in bone mineral: inorganic polyphosphate
Subsequently, the question has to be solved about the source of phosphate, required for HA formation. Initially it has been proposed that organic phosphate, in particular β-glycerophosphate, is the source of phosphate for the mineralization at least under cell culture conditions.63 However, this compound will rapidly undergo de-phosphorylation in the extracellular space, where it is also not sufficiently present to serve as a source for bone formation. In the search for a suitable origin of phosphate acting as a source we proposed polyphosphate [polyP],64 a substrate which is now well accepted.65 This polymer, polyP, exists in a polymerized state, as inorganic polyP both in a free state in serum and intracellularly in blood platelets (reviewed in ref. 65). The interesting feature of polyP is that besides providing phosphate units for Ca-phosphate mineralization, this polymer delivers chemically useful energy during enzymatic hydrolysis by alkaline phosphatase [ALP] (see below). This is the enzyme which likewise occurs also in the extracellular space and readily degrades polyP.66Following our initial observation on the presence of higher levels of polyP in bone and bone cells,64,67 it has subsequently been shown that polyP induces differentiation of osteoblasts.68,69 In turn, polyP has been fabricated as a crystalline and then as an amorphous Ca2+ salt as a scaffold for bone tissue engineering, as outlined below.70,71
2.5 Maturation of calcium phosphate to HA
Until now it has been under dispute whether amorphous calcium phosphate is indeed the precursor for the formation of crystalline HA (reviewed in ref. 28). In vitro studies suggested a transformation cascade from amorphous calcium phosphate to octacalcium phosphate and finally to carbonate hydroxylapatite;72 this pathway has been rejected for in vivo bone formation.73,74 Likewise the view that poorly crystalline carbonate hydroxyapatite acts as a precursor, needs further substantiation. In earlier studies75 the presence of an amorphous calcium carbonate (ACC) precursor phase was demonstrated in vitro.A schematic representation of the (potential) steps during bone formation is given in Fig. 1. There, also the enzymatic hydrolysis of polyP by ALP is outlined.66,76
3. Polyphosphate: a physiological inorganic polymer in higher eukaryotic cells
The physiological polymer polyP is an inorganic linear molecule which is composed of a few up to hundreds of inorganic orthophosphate monomeric units (Pi) linked by high-energy phosphoanhydride bonds.3.1 Occurrence
The natural existence of polyP, identified in yeast, is attributed to Liebermann77 and Ascoli,78 in 1890 and 1899, respectively. These authors showed that nuclear extracts contain a fraction which could be precipitated with barium salts at acidic pH, and it was termed metaphosphate. Later, this polymer was identified in bacteria, protista, flowering plants and Metazoa.79–82 Soon after the identification and characterization of ATP Lohmann, later together with Langen,83 recognized polymeric phosphate, the polyP.84–86 Only years later was the functional characterization of this molecule, especially in bacteria, pushed mainly by the groups of Holzer/Lynen, Kulaev/Belozerskij, Langen/Liss, and Kornberg.87–90 The functional characterization started with the identification of first enzymes that catalyze the reversible exchange of phosphate between polyP and ATP/ADP90–92 in bacteria. Then, again in these organisms and later in yeast a series of anabolic and catabolic polyP enzymes was discovered.93,94 While in bacteria polyP is stored in volutin granules,95 this polymer accumulates in metachromatic granules, in the acidocalcisomes, in eukaryotes. Those granules have been discovered in trypanosomes and also in human platelets.96 Besides forming salts with inorganic cations (Ca2+, Mg2+, Zn2+, Fe2+, Na+, and K+) polyP forms complexes with organic molecules, like basic amino acids or polyamines. Operationally, and based on the extraction methods used for the isolation of polyP, this polymer has been arbitrarily divided into short-chain (from 3 to 300 Pi) and long-chain (from 300 to 1000 Pi) polymers.80 Important to note is that in human platelets polyP exists as a 50 to 100 phosphate units long polymer.65The structural evidence and also the fate of polyP during the preparation can be conveniently documented by using Fourier-transform spectroscopy (FTIR) and X-ray powder diffraction (XRD).71,97 As an example the absorption spectrum, determined by FTIR, for the amorphous microparticles of the Ca2+ salt of polyP [Ca-polyP-MP] versus calcium phosphate is given in Fig. 2. The signatures for polyP are found at the wavenumbers of asymmetric (906 cm−1) and symmetric (730 cm−1) vibrations. In comparison, the asymmetric (1046 cm−1), symmetric (988 cm−1) and symmetric (899 cm−1) vibrations are indicative of the phosphate salt.
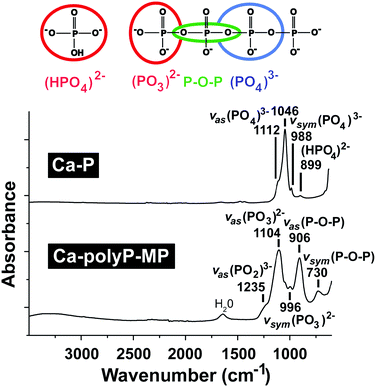 | ||
| Fig. 2 The FTIR spectral characteristics of the Ca2+ phosphate salt (Ca-P) as well as the amorphous Ca2+ polyP microparticles (Ca-polyP-MP). The typical signal peaks with their wavenumbers are given. | ||
3.2 Polyphosphate metabolic enzymes
While the anabolic and catabolic routes of polyP in bacteria are well understood81,82 only a few enzymes have been identified in eukaryotes, e.g. the major polyP-anabolic enzyme known from bacteria, polyphosphate kinase (PPK), has been identified also in the slime mold Dictyostelium discoideum.98 A polyP anabolic enzyme has been isolated with the vacuolar transporter chaperone 4 (Vtc4) in the eukaryotic yeast Saccharomyces cerevisiae.99 In mammals polyP has been identified as a substrate for the synthesis of NADP+ through phosphorylation of NAD+, a reaction which is catalyzed by mitochondrial NAD kinase.100 Then, it has been shown that in mammals post-translational modifications include polyphosphorylation events.101 Finally, data have proven that in mammals polyP is enzymatically hydrolyzed by ALP, an exopolyphosphatase, starting the cleavage from the terminal phosphate of the polyP chain with the release of Pi.66 It could be established that the ALP in the bone-tissue associated epiphyseal cartilage is able to cleave both phosphorolytically and pyrophosphorolytically within phosphate esters as well as within ATP and also inorganic pyrophosphate.102 Finally, a human exopolyphosphatase has been described, the DHH superfamily human protein h-prune, a binding ligand of the metastasis suppressor nm23-H1 protein; this enzyme degrades polyPs of all chain lengths.103 Even though the underlying enzyme has not been isolated, experimental results indicate that polyP is degraded in the human blood or plasma both by exo- and by endopolyphosphatase(s).1043.3 Polyphosphate nano-/microparticles
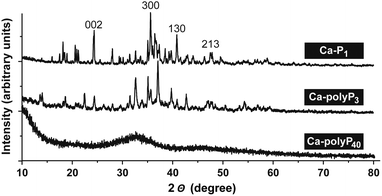
Solid evidence exists that polyP is intracellularly stored as amorphous Ca2+-polyP granules in the evolutionary highly conserved intracellular granules.105,106 In a biomimetic approach our group succeeded in preparing amorphous Ca-polyP microparticles (Ca-polyP-MP).71 The concentration ratio between Ca2+ and polyP was found to be crucial for obtaining those particles; the weight ratio between Ca2+ and polyP should be ≥2-fold. In addition the pH must be maintained throughout the preparation at 10. The final drying temperature of the particles is performed between 60 °C and 80 °C. In more detail, 28 g of CaCl2·2H2O (Sigma, Taufkirchen; Germany; ≥99%), dissolved in 250 ml of water (pH 10), are added to 10 g of Na-polyP, with an average chain length of 40 phosphate units (Chemische Fabrik Budenheim, Budenheim, Germany; developmental product, for use in foodstuff-powder E 452 food grade). Then the microparticles formed are collected by filtration, washed three times with ethanol and subsequently dried in an oven at 60 °C.The X-ray diffraction [XRD] spectrum for Ca-polyP40 indicated that the particles are amorphous (Fig. 3). However, if for the preparation of the microparticles Na-polyP is replaced by Na-phosphate (Sigma; 96%) or by Na-tripolyphosphate (Sigma; 85%) the resulting particles, Ca-P1 and Ca-polyP3, are crystalline. The dominant, characteristic peaks107 are marked in the recorded pattern (Fig. 3).
It should be noted that the formation of crystalline Ca-phosphate is a pathological process especially in the joints and led to the use of the term “crystal deposition disease”.108,109 Physiologically crystal formation in the human body is prevented by proteins present in the plasma.110 As an example, fetuin-A, a well-known circulating serum glycoprotein, is a potent systemic calcification inhibitor; those proteins inhibit crystal formation by binding to Ca2+ phosphate.
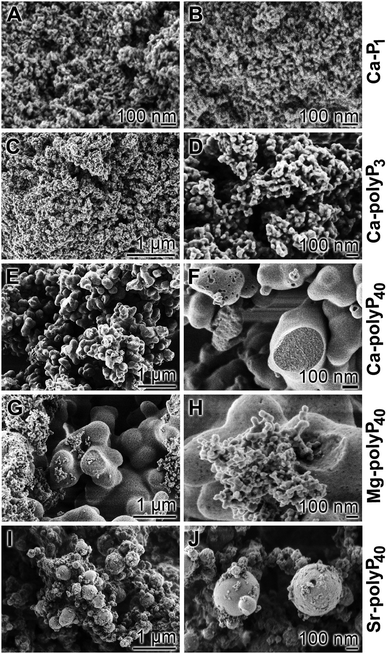
Analysis of the Ca2+ deposits formed by the different phosphates, using a scanning electron microscope [SEM], shows that the Ca-P1 particles (Fig. 4A and B) have a size of around 25 nm, while those of Ca-polyP3 have a size of approximately 40 nm (Fig. 4C and D). In contrast the amorphous Ca-polyP particles show no signs of crystal formation (Fig. 4E and F). Their size varies between 80 and 200 nm;71 a close range can be obtained by an exact setting of the desired pH value between 9.0 and 10.0. From studies with Ca2+ phosphate and carbonate nanoparticles it is known that the crystallization can pass through a gelly-like phase, the coacervate.111,112 Such a phase is formed during an electrostatically-driven liquid–liquid transition phase, resulting from the association of oppositely charged (macro)-ions, the negatively charged polyP and the water layer. Those coacervate assemblies can measure >100 μm and are formed from their soluble precursors of less than 200 nm.113 The amorphous Ca-polyP particles, with a chain length of around 40 Pi units, are not solid but comprise a porous internal structure with a labyrinth-like canal system of ≈20 nm dimensions. If particles are formed from MgCl2·6H2O or SrCl2·6H2O, respectively, the morphologies of the polyP particles are less homogeneous.114,115 As shown in Fig. 4G and H the Mg-polyP particles can reach sizes of 1 μm or 50 nm; a similar size range is seen for Sr-polyP (Fig. 4I and J). A more narrow distribution of the particles is obtained if the pH in the aqueous system is strictly adjusted to 10 using a pH-stat system. The amorphous particles prepared from polyP have a similar size-distribution to those particles seen in the acidocalcisomes and are also amorphous like them.116
3.4 Amorphous Ca2+ orthophosphate particles
The salt from Ca2+ and orthophosphoric acid is crystalline.117,118 Interesting enough the group of Tas described a procedure to fabricate amorphous Ca-phosphate spheres by introducing a procedure including simulated/synthetic body fluid (ref. 119, reviewed in ref. 120). In this “physiological” approach they added to CaCl2·2H2O the hydrogen phosphate Na2HPO4 in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 weight ratio in the presence of an excess of NaCl and MgCl2 and also of lactic acid. They adjusted the pH to 7.4 and kept the processing temperature below 70 °C. After stirring and washing they collected amorphous particles of size 245 nm. This procedure allowed the inclusion of gelatin which also gave amorphous microparticles.119 This novel approach allowed also the inclusion of other ions, like Mg2+, present in large amounts in the blood plasma. Those amorphous Ca-phosphate particles have been proposed to build with collagen fibrils biocompatible hybrid scaffolds, following the biomimetic route.120
1 weight ratio in the presence of an excess of NaCl and MgCl2 and also of lactic acid. They adjusted the pH to 7.4 and kept the processing temperature below 70 °C. After stirring and washing they collected amorphous particles of size 245 nm. This procedure allowed the inclusion of gelatin which also gave amorphous microparticles.119 This novel approach allowed also the inclusion of other ions, like Mg2+, present in large amounts in the blood plasma. Those amorphous Ca-phosphate particles have been proposed to build with collagen fibrils biocompatible hybrid scaffolds, following the biomimetic route.120
With these data at hand studies will next be initiated to prepare polyP microparticles from Na-polyP and CaCl2 but also co-adding additional ions like Mg2+ and lactate. This rational is also based on the pioneering studies of Bachra et al. that disclosed artificial/simulated biological fluids of pH 7.3 and ionic strength 0.16 M to facilitate the formation of amorphous Ca-phosphate deposits. The transformation into cryptocrystalline material is controlled by Mg2+ and HCO3−.121–124 Even more this group reported the first studies on the co-preparation of amorphous Ca-phosphate particles with collagen.125,126
4. Polyphosphate: an extracellular store of metabolic energy
All living systems have to maintain a steady-state which is characterized by a thermodynamic cycle in which the entropy of the system is lower than that of its nonliving environment which proceeds with an increase in entropy.127 Furthermore, all forms of transformations of energy, including entropy and free energy at a given temperature, follow the laws of thermodynamics.128 In turn, to understand the process of energy transformation two arms have to be considered that are required to drive metabolism in living systems: (1) energy conservation and (2) energy dissipation. It is inherent that during these processes a portion of energy is lost as heat. The term free energy (ΔG; Gibbs free energy) describes the gain or the loss of energy during a reaction and is calculated as ΔG = ΔH − (T × ΔS) [where H is the enthalpy, T is the temperature and S is the entropy]. Following the basic reflections by Lippman129 the free energy that is produced in a living system during catabolic metabolic reactions is stored in energy-rich compounds, like ATP. In turn, the stored free energy can subsequently be re-used to drive endergonic reactions required to run and maintain anabolic reactions. The breakdown of “foodstuffs” results in the formation of “free energy”, a special kind of chemical energy, which is then converted into other forms of energy, for example, mechanical work (muscle), osmotic work (secreting glands) or heat (fat tissue). This scientific evidence implies that during enzymatic hydrolysis of high-energy phosphate bond(s) in ATP [e.g. by ATPase(s)], or ADP [e.g. ADPase(s)] biochemically useful/convertible energy is released, while a portion is converted into heat.4.1 Generation of ADP/ATP
The formation of bone mineral deposits occurs mainly or exclusively in the extracellular space.130 In this compartment ATP-consuming kinases are crucially involved in the control of certain metabolic events, for example, phosphorylation of secreted proteins controlling biomineralization, not only intracellularly, but also extracellularly; among them is the extracellular Fam20C kinase that phosphorylates S-x-E/pS motifs in the ECM (extracellular matrix) of bones.131 In addition, a secreted protein tyrosine kinase, VLK, has been identified that phosphorylates essential proteins during embryonic development.132 No distinct motif for this class of enzymes has been identified yet. In general terms, the binding sites for ATP or ADP follow the very common and ancient signatures existing from the origin of life; with GxGxxG and GxxGxG and ending with a distinct signature for the specific ligands.133 Interestingly enough, clusterin an extracellular chaperone that is involved in the sequestration of the oligomeric forms of the Aβ1–40 peptide134 also shares this motif.135 Finally, extracellular ATP acts as an excitatory transmitter during synaptic transmission after binding, e.g., to purinergic receptors, ecto-ATPases or through phosphorylation via ecto-protein kinases136 as in blood platelets.137 Then, ATP has been shown to be involved in the modulation of cell–cell spreading of Ca2+ signals between mast cells (see: ref. 138) and also in blood platelet aggregation.139Like ATP, polyP contains high-energy phosphate units, linked via energy-rich phosphoanhydride bonds;76,140Fig. 5. In order to screen for a potential pathway that allows the generation of ATP from polyP, adenylate kinase ([AK]; EC 2.7.4.3) has been addressed at first.76 AK is known to act with phosphotransferase activity that catalyzes the interconversion of adenine nucleotides and plays an important role in cellular energy homeostasis. Seven AK enzyme isoforms have been described in mammals (reviewed in ref. 142), among them are the muscle AK1 and AK2 isoforms which are located at the inner and outer cytosolic and mitochondrial membranes, AK3 which is a GTP:AMP phosphotransferase of intra-mitochondrial AMP, and AK4 and AK5 which are located in the mitochondrial matrix of neuronal and pancreas cells. In addition, AK6 which has been identified in the cell nucleus exists that might have a role in the nuclear energy provision, and finally AK7 which occurs in epithelial cells. The most likely candidate for the extracellular phosphotransferase reaction is AK1 which undergoes secretion into the extracellular space.143 This enzyme, most likely the isoform AK1β, has also been proposed to mediate the synthesis of ATP which acts as a crucial mediator of chemosensory transduction.144 Experimental evidence has been presented showing that AK1 secretion is required for the accumulation of extracellular ATP, as in myotubes.143
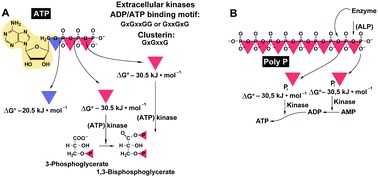 | ||
| Fig. 5 Energy-rich bonds. (A) ATP comprises two high-energy phosphoanhydride bonds (red triangles) and one ester phosphoester bond (blue triangle). This nucleotide can bind to enzymes (like kinases), while ADP with its ADP-binding motif can (potentially) react with the chaperon clusterin. (B) In the linear inorganic polyphosphate (polyP) up to 1000 phosphate (Pi) residues are likewise linked with high-energy phosphoanhydride bonds. Those bonds are prone to hydrolysis by enzymes (like the ALP) with the release of the “stored” energy (ΔG: −30.5 kJ mol−1), which in turn can mediate the transfer of the high-energy phosphate to other metabolites. (Partially from ref. 141 [with permission].) | ||
As mentioned, polyP is enzymatically hydrolyzed by an ALP.66 In turn, and following the Lippman concept of free energy, the energy stored in the energy-rich phosphoanhydride bonds formed during the anabolic and energy-dependent reactions145 will be released as free energy and subsequently transformed into chemical energy (bond energy), prior to be converted into other forms of energy, such as mechanical work in muscle or osmotic work in secreting glands.146 Based on our findings that polyP upregulates both intracellularly and also extracellularly the ATP and ADP pool size in the bone-related SaOS-2 cells140 we dissected the extracellular enzymatic energy flow by using the natural inhibitor of the AK enzyme(s), A(5′)P5(5′)A.147 Under these conditions, the extracellular ATP pool size decreases in the presence of the inhibitor in favor of ADP;76Fig. 6A. Considering the finding that ATP is released by bone cells via vesicular exocytosis,148 the experiments were performed in the presence of N-ethylmaleimide and brefeldin A,149,150 inhibitors which did not change the results significantly. In the absence of A(5′)P5(5′)A, polyP caused a strong upregulation of the ATP pool;76Fig. 6B. From these experiments we deduce/postulate that the ALP-mediated hydrolysis of the anhydride bonds results in a phosphorylation of AMP to ADP, via an enzyme not yet known, and subsequently a further AK-driven phosphorylation of ADP to ATP.76
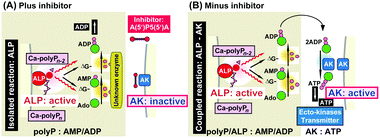 | ||
| Fig. 6 Schematic outline of the proposed polyP-driven phosphorylation of AMP to ATP via ADP. (A) In the uncoupled reaction (in the presence of the AK [adenylate kinase] inhibitor A(5′)P5(5′)A) the polymer undergoes extracellular hydrolysis via ALP [alkaline phosphatase] under simultaneous increase of the ADP pool. (B) In the absence of A(5′)P5(5′)A the polymer is hydrolyzed by ALP and the metabolic energy, released during cleavage, is stored in ADP which is then converted to ATP and AMP. (Partially taken from ref. 76 [with permission].) | ||
With these data gathered it appears that a gap in the understanding of the energy balances has been closed. With the discovery/identification of mitochondria,151 the identification and characterization of the triphosphate ATP84,85 and finally the transport mechanisms of ADP and ATP with their carriers from the mitochondria (reviewed in ref. 152) it became overt that the balance between energy production and expenditure is linked with ATP; the ratio of the adenylate pools determines the energy charge of the cell.153
In the extracellular space the ATP concentration, as a signaling molecule in mammals, is comparably low. While in the cytosol the ATP concentration is in the range of 3–10 mM, the extracellular level – under basal conditions – is approximately 106 times lower.154 Studies suggested that the cytosolic ATP provides the source for extracellular ATP.155
4.2 The extracellular store
The mechanism of ATP release from the cells is not sufficiently understood; a wide range of stimuli have been proposed (reviewed in ref. 156). However, a thorough calculation of the energy consumption in the extracellular space has to be performed and the turn-over rate of ATP in this compartment needs to be studied. In this context a comparison between the level of extracellular ATP and polyP should be given. The highest concentration of polyP is found in the blood platelets (average chain length of 70–75 Pi units); there, polyP is synthesized with a concentration of 0.74 nmol/108 platelets (reviewed in ref. 157). If this figure is used to calculate the total concentration of polyP in those cells a value of approximately 1.1 mM results. By this, the level of polyP is 10 to 20 times higher than those measured in other mammalian cells.158 Intracellularly polyP is concentrated almost completely in “dense granules” organelles that share morphological and biochemical similarities with acidocalcisomes. In turn, the concentration of polyP in those granules is 130 mM, if expressed in terms of orthophosphate (Pi). The polymer is released from the platelets after activation of these cells105,159 and becomes accumulated in the blood at a concentration (human) of 1 to 3 μM (a total of 5.5 to 16.6 μmoles in human blood). In turn, the absolute concentration of polyP in the blood is considerably higher than that of ATP. In two general schemes (Fig. 7) it is outlined that the intracellular energy is, generally speaking, provided by ATP as high-energy phosphate, while glucose and others, like lactate, are the energy stores (Fig. 7A). In the blood the exchange of high-energy phosphate to the effector organs is insignificant. In contrast, in the extracellular space polyP is dominant as an energy-rich polyP over ATP and transported via the blood platelets (Fig. 7B).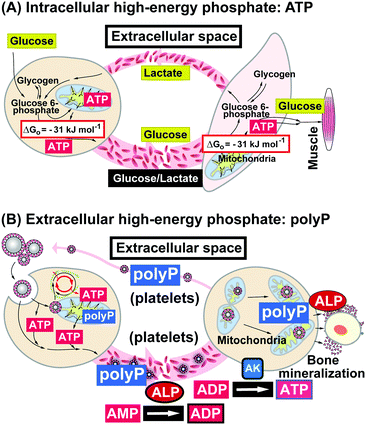 | ||
| Fig. 7 Generation and transport of high-energy phosphates in the two compartments. (A) Focusing on the intracellular distribution, the metabolites, e.g. glucose or lactate (besides lipids or carbohydrates), are transported via the bloodstream and are shuttled between the primary, secondary and finally the effector cells; there they are metabolized intracellularly as a source for high-energy phosphates, ATP; this metabolite remains (almost) completely in the cells. (B) In contrast, polyP is the extracellular high-energy phosphate and shuttles like this between cells within the acidocalcisomes, especially in the blood platelets. In the extracellular compartment metabolic energy transfer from polyP to ATP via ADP is mediated by ALP (release of metabolic energy from the acid anhydride hydrolysis) and AK. (Partially taken from ref. 157 [with permission].) | ||
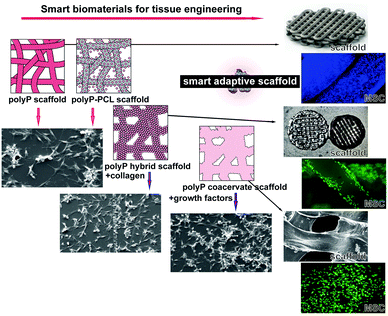
5. Smart biomaterials
As outlined above the basis of any kind of regeneratively active biomaterial, to be applicable for implant formation, should be a material that “actively participates in the formation of functional tissue”.5 However, it should be distinguished between a biomaterial itself and a scaffold which is qualified by the label “smart”.A smart scaffold can be a hybrid material, functioning by adjustment of the physical properties of the scaffolds, inclusion of ECM motifs in the scaffolds and finally the inclusion of active substances into those, largely inert scaffolds.160
It is indicative that blood platelets have been used since years to treat patients with thrombocytopenia to restore hemostasis. Furthermore, platelets have been implicated in immune responses and tissue repair, e.g. wound repair, associated with inflammation, cell proliferation and differentiation, and remarkably also in tissue regeneration. Special focus has been placed on the interactions with progenitors and control of apoptosis/cell survival. Based on an overwhelmingly large volume of data on the effect of platelet-rich plasma on any kind of tissue repair it has been concluded that platelets are fundamentally involved in repair and regeneration of damaged tissues and preservation of organ function (reviewed in ref. 161 and 162). The major elements within the platelets causing this beneficial effect during regeneration have been seen – surely well-founded – in the effects of the biologically active organic mediators which are released by those cells into the environment like the various cytokines, chemokines, and growth factors. With the discovery that polyP, as one major, even inorganic, polymer of the platelets being functionally active during bone regeneration,64 growing evidence has been presented that this bio-inorganic polymer has the potential to gain an increasingly dominant position in regenerative medicine.79,157 Basically, polyP is a structurally very simple linear polymer composed of Pi units that are linked by high-energy phosphoanhydride bonds. At neutral pH the phosphate units are monovalently negative charged; the polyanion can form salts, among them are the physiological cations Ca2+, Mg2+ and Sr2+ (reviewed in ref. 80). Commercially available, on a large scale, is Na-polyP. As outlined below the biological activity, as well as the application of polyP, are strongly dependent on the cation used for salt formation (Fig. 8).
Ca-polyP-MP are degraded by the enzyme ALP.71 Those particles are smart since the degradation rate can be expected to be different in non-regeneration bone regions versus actively regenerating zones. This conclusion can be drawn from the observation that ALP is upregulated during regeneration.163 Besides this dynamic adaptation of the release kinetics, followed by a modulation of the energy release and phosphate provision, processes required for bone formation, the particles can be loaded with polymer salts comprising the different counter-cations. By this, the “smart scaffold” to be implanted can be adjusted from bone (Ca2+ or Sr2+ polymer) to cartilage (Mg2+ salt), according to the environment in which it is used, either with more osteogenic or more chondrogenic differentiation activity to the stem cells. Thus the “smartness” of the polyP-based biomaterial is not only restricted to surface modifications, like a shift to nanoparticles or the addition of ECM-like molecules,164 but is a genuine property of the biomaterial in toto, initiating and maintaining the natural regenerative process at the damaged site.
6. Amorphous polyP particles (microparticles/nanoparticles) in tissue engineering
The majority of the studies with amorphous Ca-polyP microparticles, as well as nanoparticles, have been performed with a formulation, prepared from Na-polyP, with a chain length of 40 Pi units and CaCl2 at a pH of 10. Those smart particles, prepared in a bioinspired way, were tested for their applicability as implants in the field of tissue engineering with a special focus on osteo-articular tissues.In tissue engineering strong attempts have been made to functionalize scaffolds with biological ligands that enhance the biocompatibility and bioinducibility of the usually synthetic, largely biologically inert scaffolds or matrices. Those materials had to be used since the natural materials, even though biocompatible and provided with suitable adhesive sequences supporting cell adhesion and cell differentiation, possess inadequate mechanical properties, are rapidly degraded and have variable properties, depending on the extraction procedure used (reviewed in ref. 165). Furthermore, they possess the inherent risk of contamination and have high production costs. In contrast, synthetic materials can be fabricated with suitable mechanical properties, are of highly reproducible chemical and mechanical compositions, of low production cost and elicit only low immune responses. Their disadvantages are that they have only low biocompatibility and are prone to biodegradation with concomitant side effects.165
Among the existing scaffolds, the polymer polyP has many advantages as it:
– is natural,
– is bioinorganic,
– is of low cost,
– can be prepared in a standardized production line,
– is biodegradable,
– has mechanical properties of the physiological hard/semi-hard tissue,
– is biocompatible,
– elicits no immune reactions,
– is morphogenetically active,
– can be used for bioprinting,
– can be additionally functionalized with bioactive peptides,
– can be used as cage for drug delivery.
A few key characteristics might be mentioned here.
6.1 Biodegradability
As any bio-polymer polyP is degradable. It is prone to the enzyme ALP which is required for the release of the metabolic energy of the high-energy acid anhydride linkages.76 The important issue here is that the polyP particles have high stability in water or solutions, supplemented with mineral components. Only if in contact with organic fluids, like blood plasma, do the dissolved peptides/proteins cause an accelerated hydrolysis.In order to prevent a fast enzymatic decay during in vivo experiments the polyP particles can be encapsulated into PLGA [poly(D,L-lactide-co-glycolide)].166
6.2 In situ hardening
As a polyanion polyP is negatively charged under physiological conditions and hence can be combined with hydrogels, like alginate or hyaluronic acid,115,167 and processed. After the fabrication step the organic–inorganic hybrid material can be exposed to Ca2+ ions that link the polymers together via non-covalent ionic linkages. Based on the exposure time and the molarity to this cation the material can be hardened to strengths suitable to be used as implants for bones,166 cartilages,115 or organic mats.1686.3 Biocompatibility and zeta potential
A basic and an absolutely essential property of implants/scaffolds for tissue engineering is their biocompatibility. The cells must have a strong tendency to adhere to the inserted material and allow a normal functionality, including migration onto the surface, spreading, proliferation and differentiation.In analogy to the dynamic biofouling process, occurring on the surface of hard/solid surfaces of synthetic polymers in the aqueous environment (reviewed in ref. 169), the steps of interaction between implants and biological fluids/tissues have been subdivided into six stages.170 The osteoconductive171 stage, a passive process during which the biomaterial becomes integrated by bone forming cells in the grafting area, proceeds in six stages at the interfaces between the implant and the bone defect; (i) nano-/microparticle and serum adsorption and deposition, (ii) cell attraction and recruitment, (iii) adhesion, migration as well as proliferation of osteogenic cells, (iv) cell differentiation and bio-seed formation, (v) matrix calcification, and finally (vi) bone remodeling. During phase 1, the key regulation of the subsequent colonization with cells, a conditioning layer is formed through adsorption of organic molecules by the fluid-exposed surface; in phase 2, the colonization/adsorption of bone cells, and also of microorganisms in patho-physiological situations, occurs. In phase 3, cell adhesion is primarily controlled by two properties, the surface characteristics of the respective biomaterials and the consecutive extracellular stimulation of the cells by the topography, functional groups, and wettability. In particular, in biomaterials with regeneration-inducing potency phases 4 to 6 become dominant and beneficial and induce cell differentiation and bio-seed formation, calcification, and bone remodeling.
The zeta (ζ) potential of the implant surface is crucially important for the process of cell interaction with an implant material. This highly dynamic parameter reflects the surface charge when an implant is brought into contact with the aqueous environment. The zeta potential is the resultant of the separation in charge between the solid phase of the implant or the bone and the aqueous surrounding milieu. It has been determined that the zeta potential of a biphasic calcium composite material is approximately −20.4 mV172 and of non-modified titanium around −10 mV.173 Usually it is accepted that the increase of the negativity of the zeta potential of an implant allows a more favorable integration of the material into the tissue (e.g.ref. 174). In the measurements, performed by us at pH 7 (not published), we found that hydroxyapatite shows a zeta potential of −5.09 ± 1.2 mV, while the nano-/microparticles prepared from Na-polyP reached a zeta potential of −42.3 ± 5.3 mV (hydrodynamic radius between 307 and 859 nm) and −33.6 ± 2.3 mV (61–198 nm). This finding implies that the polyP nano-/microparticles have a lower tendency to aggregate, compared to hydroxyapatite. The zeta potential is dependent on the presence of ionic polyelectrolytes. In any event, adsorbed polymeric macromolecules, like proteins, reduce the zeta potential concomitantly with a shift of the slipping plane from the solid surface of the particles. The effect of counter-ion displacement influences the zeta potential even to a higher degree and is also dependent on the charge of the colloidal particles.175
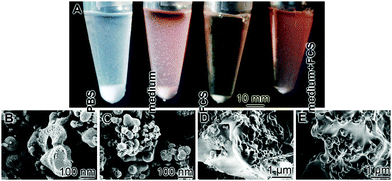
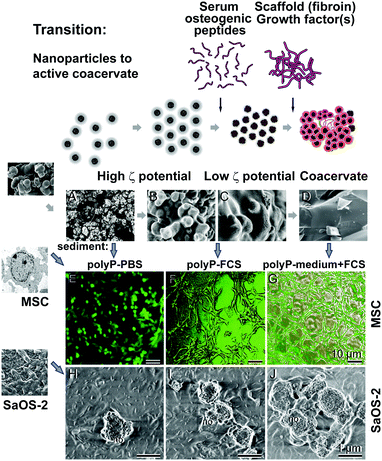
The zeta potential also controls the efficiency and the selectivity of the peptides that can be adsorbed by the respective particles.175 During this process the zeta potential decreases, the particles aggregate, and coacervation can proceed. This process can be accelerated by a reduction of the dielectric constant by heat or e.g. by methanol.176 Focusing on polyP these authors found that during water removal and the reduction of the dielectric constant, the approximation of the polyphosphate chains occurs resulting in the coacervation process. During this phase transition a destabilization of the aqueous layers between the polyP chains occurs resulting in a decrease of the electrostatic repulsion between the polyphosphate chains. In our studies (unpublished) we found that after transfer of Ca-polyP-MP into cell culture medium, supplemented with 10% fetal calf serum [FCS], the particles remove protein(s) out of the aqueous environment and hence the sedimented pellet increases in weight, followed by the coacervation phase (Fig. 9A); the duration of this study was 7 d. The Ca-polyP-MP cannot adsorb protein from a medium lacking FCS, or from PBS, and remain suspended. A subsequent transfer of the polyP-based coacervate to increased temperature (80 °C), followed by water removal, initiates nanoparticle formation. A SEM analysis supports this observation. After keeping the Ca-polyP-MP for 7 d either in PBS (phosphate buffered solution) or in medium, the microparticles remain in the isolated suspended state (Fig. 9B and C). In contrast, if the particles were suspended in FCS, or in medium with 10% FCS they fused to coacervate deposits (Fig. 9D and E), and almost no isolated particles could be detected in the solid material.
6.4 Immune reactions
Until now no immune reactions have been reported for polyP. It has been proposed that polyP might elicit proinflammatory responses through the activation of the NF-κB pathway, perhaps reflecting the proinflammatory properties (proinflammatory mediators) of activated platelets.1776.5 Morphogenetic activity
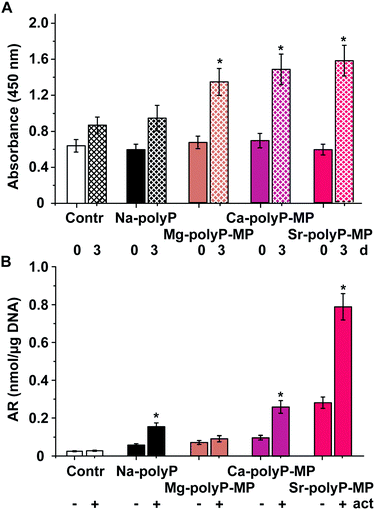
The impressive and important property of the polyP microparticles, which distinguishes those particles from (almost all) other scaffold materials is that, in dependence on the type of the counterion (cation) [Ca2+, Mg2+ or Sr2+] used during the formation of the amorphous particles (Fig. 8), a more specific biological response could be determined. All four particles were found to be amorphous, as determined by XRD analysis.71,114,115 The formation of nanoparticles is not only restricted to bivalent cations, but can also be extended to a trivalent gadolinium ion. Also this cation allows the formation of amorphous polyP nanoparticles/microparticles.178 A comparative in vitro study with SaOS-2 cells is shown in Fig. 10.SaOS-2 cells are bone-related tumor cells179 that readily form hydroxyapatite in vitro.69 As an activation cocktail for mineral deposition the components 5 mM β-glycerophosphate, 50 mM ascorbic acid and 10 nM dexamethasone were used.180 At first the effects of the different particles, 10 μg ml−1 of Na-polyP (Ca2+-complexed), Mg-polyP-MP, Ca-polyP-MP or Sr-polyP-MP were assayed for viability/growth on SaOS-2 cells. In the controls, no polyP was added. As seen a significant effect on growth by the particles is found during an incubation period from time 0 (0 d) to day 3 (Fig. 10A). While for the assays with Na-polyP no significant increase in viability is seen with respect to the controls, the three assays, supplemented with Mg-polyP-MP, Ca-polyP-MP or Sr-polyP-MP showed a significantly enhanced growth compared to the polyP-free controls. Among the particle formulation themselves, no significant difference could be measured.
The inducible effect is not only restricted to the viability/growth of the cells but also to the activity of mineral deposition. Mineral deposition was determined by the spectrophotometric assay using Alizarin Red S stain.181 The amount of bound Alizarin Red S was normalized to the total DNA in the samples. At first the cells were incubated again with 10 μg ml−1 of Na-polyP (Ca2+-complexed), Mg-polyP-MP, Ca-polyP-MP or Sr-polyP-MP, or remained without polyP (control) for an incubation period of 3 d. Then the activation cocktail was added to the cultures, which were subsequently incubated further for 5 d. Then the extent of mineralization was determined. Using this approach it is evident that a significant increase of mineralization occurred in the assays with Na-polyP (Ca2+-complexed), Ca-polyP-MP or Sr-polyP-MP. Remarkable is the stimulatory effect displayed by Sr-polyP-MP (Fig. 10B).
Interestingly no significant effect is measured in the assays with Mg-polyP-MP (Fig. 10B). This finding, together with the observation that Mg-polyP-MP (Fig. 4G and H) have a broad size distribution and share similarity to the coacervate aggregates, led to the assumption that the coacervate state co-determines the biological effect of polyP. In turn, Ca-polyP-MP incubated for 7 d only in PBS, or in FCS, or in medium plus FCS was tested for the morphogenetic activity using MSC. After incubation the three samples were obtained by centrifugation and added at the same concentration (50 μg ml−1) to cultures of human MSC or SaOS-2 cells.182 The polyP deposits remaining after incubation in PBS (polyP-PBS sediment) hardly changed the arrangement of the MSC on the plates during a 24 h-incubation (Fig. 11E). In contrast, the polyP aggregates, formed in the presence of FCS (polyP-FCS sediment) or in the assays with medium plus FCS (polyP-medium + FCS), prompted the cells to migrate and to form a pattern of attaching and communicating cells (Fig. 11F and G). Even more striking was the effect of the different polyP deposits on the mineralization activity of SaOS-2 cells. During an incubation period of 3 d in the presence of 50 μg ml−1 of polyP-PBS only occasional mineral nodules are seen on the cells (Fig. 11H), while in cultures supplemented with either 50 μg ml−1 of polyP-FCS or polyP-medium + FCS densely arranged mineral nodules are seen on the surface of the cells (Fig. 11I and J). This piece of study strongly suggests that polyP microparticles change their biological potency in dependence on the state, particle form or coacervate aggregate.
This described observation in the work leads to another important consequence. As outlined above, peptides and/or proteins added to polyP microparticles/nanoparticles, originating from serum or in the form of synthetic peptides, like osteogenic peptides derived from BMP-2,183 or structural scaffold materials like fibroin,184 become readily incorporated into the particles due to their high ζ potential (Fig. 11), resulting in the formation of coacervate deposits under physiological conditions (Fig. 11A–D). A likewise straightforward inclusion of structural proteins, like fibroin, can be achieved with Ca-polyP microparticles (Fig. 12). The fibroin is extractable from Bombyx mori raw silk fibers (Fig. 12A) purchased from Suzhou Silk Factory (Jiangsu; China). The fibers were degummed184 using the Na2CO3 procedure. The final soluble product was dialyzed against deionized water and added at a concentration of 3 wt% to a 20 wt% suspension of Ca-polyP microparticles (Fig. 12B). In addition, 1.5 g of solid CaCl2 was added. The resulting gum-like material was moldable (Fig. 12C): after thorough washing in PBS the plain surface (Fig. 12D) provided a suitable platform for MSC. After a 2 d incubation the cells attached and migrated to each other and proliferated (Fig. 12E). The fibroin internal scaffold is durable and could be released from the polyP surrounding material after a 7 d period (Fig. 12F).
The morphogenetic activities of the three major forms of the polyP microparticles/nanoparticles are outlined in the next section.
7. Studies on the potential use of amorphous polyP particles for bone/cartilage repair
Following our studies, summarized above which revealed that polyP apparently is used as a source material for the deposition of hydroxyapatite from amorphous Ca-phosphate it appeared to be reasonable to study first the potential application in orthopedic tissue engineering.7.1 Activity of the various slowly soluble polyP salts in the long bone assay
The three salts of polyP from which amorphous nano/microparticles had been prepared have been further developed towards the application of bone and cartilage repair. The major cells involved in the repair of these hard/semi-hard tissues are the MSC from which the two cell lineages towards the osteoblasts for bone formation and chondrocytes for cartilage synthesis originate.185 Besides these two finally differentiated cells, two further lines differentiate from the MSC: the tenocytes, which are elongated fibroblast type cells that give rise to the tendon cells, and the myocytes that form the muscles.186 Depending on the signaling molecules they are exposed to186 the inherent potential of the MSCs is to exhibit a positive role in tissue repair and tissue regeneration, especially in the field of bone-healing problems and early stages of osteo-articular impairments.187Recently, an approach has been summarized to use almost complete femur explants from mice as an in vitro model to study the effect of amorphous polyP particles on the differentiation of the cells of the bone marrow in their natural microenvironment with major emphasis on the osteogenic and chondrogenic lineages (Fig. 13A and B). The expressions of the differentiation/transcription factor SOX9 (differentiation towards osteoblasts) and of the factor RUNX2 (chondrocyte differentiation) were used as markers;188Fig. 13. The steady-state-expression of both marker genes was found to be upregulated after exposure to Ca-polyP microparticles. Interestingly, the genes for the bone-catabolic enzymes in the osteoclast, cathepsin-K became down-regulated in this system. However, this system appears not to be applicable for more detailed studies to determine the requirements of the polyP particles as a suitable scaffold in tissue engineering. Therefore, cell culture and animal experiments were performed with those materials.
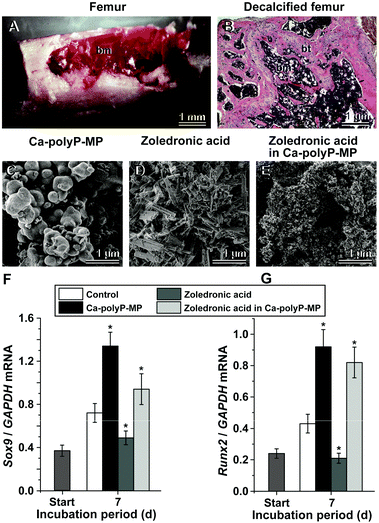 | ||
| Fig. 13 Application of polyP as a drug carrier in the long bone assay. The long bone, mouse femur, model was used to demonstrate the bi-functional effect of polyP, co-precipitated with zoledronic acid in the presence of Ca2+. (A) Dissected and prepared mouse femur for in situ incubation of bone cells (bone marrow [bm]) within the femoral bone cavity. (B) The bone had been decalcified with EDTA (ethylene diamine tetra-acetic acid) and then stained with hematoxylin-eosin, to distinguish between the bone tissue (bt) and bone marrow (bm). Morphology of the polyP and zoledronic acid particles; SEM images: (C) amorphous Ca-polyP microparticles, (D) crystalline Ca2+ salt of zoledronic acid, (E) amorphous hybrid zoledronic acid in Ca-polyP microparticles. Steady-state-expression studies of bone marrow cells after exposure to amorphous Ca-polyP microparticles, crystalline Ca2+ salt of zoledronic acid, and amorphous hybrid zoledronic acid in Ca-polyP microparticles (30 μg ml−1 each): effect of the compounds on (F) chondrocyte differentiation [expression of the Sox9 marker gene] or on (G) osteogenic differentiation [Runx2 gene]. The expressions were determined by PCR after 7 d.188 Standard errors of the means are shown (n = 6 experiments per time point). The significant differences between the values in the controls and the respective treated samples are indicated with asterisks; * p < 0.01. (Partially taken from ref. 188 [with permission].) | ||
7.2 Characteristics of Ca-polyP-MP for bone implants
The first form of the particles described and being amorphous has been obtained with the Ca2+ salts from polyP.71Biodegradability: the particles are degradable via ALP in the same process as described for Na-polyP.66,71
Biocompatibility: the initial study was performed in rats166 with amorphous Ca-polyP particles that had been encapsulated into PLGA. Those spheres were inserted into critical-size calvarial defects.166 The results revealed that those particles are superior to β-tri-calcium phosphate (β-TCP) controls and initiated a faster regeneration.
Regeneration activity – including osteoinducibility [potency of the MSC to stimulate towards the bone-forming cell lineage (according to ref. 171)] and osteoconduction [facilitation of bony in-growth/guiding the reparative growth of the natural bone]: the animal/calvarial defect studies revealed that the polyP-based implant material is superior to microspheres filled with β-TCP controls.
Osseointegration [anchorage of an implant by the formation of bony tissue]: this property was met by the characteristics just described.
Mechanical properties: these properties are not crucial for the sphere (diameter ≈ 800 μm)-based implants, applied for the small defects. The regeneration area (after the 12 week healing period) showed for the polyP-based implant a Young's modulus of 1.74 MPa, while for the β-TCP-controls only a value of 0.63 mPa was reached;166 in parallel studies the modulus for the surrounding trabecular bone tissue was found to be only slightly higher with a value of 3.05 MPa.
Morphogenetic activity [control of cell growth and cellular differentiation through differential gene expression]: the ALP gene expression is considered as a major marker for a physiological bone mineralization process (reviewed in ref. 189). This gene becomes strongly upregulated in the presence of Ca-polyP particles.62 In contrast, the expression of the membrane-associated carbonic anhydrase IX, an indicative gene for Ca-carbonate bioseed formation, remains unchanged.166 A further gene anabolically implicated during bone formation, collagen type I190 is also strongly upregulated in the presence of these particles, while β-TCP caused no effect.191
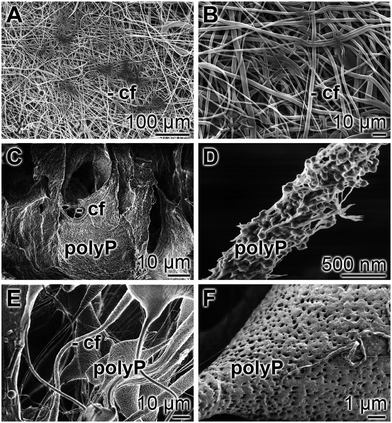
Scaffold architecture: for small defects the sphere technology has been applied until now. In anticipation of future animal studies a preparation technology has been introduced for larger implants. The freeze-extraction technology, using polyP together with collagen, was introduced to meet the requirements of a suitable architecture of scaffolds.192 Such an implant material provides an interconnected pore structure with high porosity to allow cellular penetration and physiological diffusion of nutrients to the cells within the implant material.193
Manufacturing technology: the material, Ca-polyP microparticles/nanoparticles, is cost-effective. The starting polymer, Na-polyP, is extensively purchasable and affordable (inquiry at: Chemische Fabrik Budenheim, Budenheim, Germany). Since it is a synthetic polymer it can be produced in a scalable process also achieving good manufacturing practice standards. The particles and the spheres are durable and can be used in practice with usual tools.
7.3 Acceleration of bone repair by Sr-polyP
Strontium cations serve as suitable counterions and allow the formation of Sr-polyP microparticles/nanoparticles;114,194 again a 2-fold weight excess of SrCl2·6H2O over Na-polyP allowed the formation of the 150 to 800 nm large amorphous microparticles (Fig. 4I and J). The resulting particles, Sr-polyP, strongly induced mineralization onto SaOS-2 cells in vitro and if encapsulated into PLGA also in vivo, using the rat critical-size calvarial defects as a model.114 Implants, composed of PLGA and Sr-polyP particles, caused after a 12 week implantation almost totally a regeneration of the bone defect, and restored the hardness of the new tissue to normal. It might be noted that the regeneration capacity of these particles is, in this in vivo model, superior to Ca-polyP microparticles in vitro (Fig. 10) and in vivo.114 The morphogenetic potency of the Sr-polyP in vitro is supported by the results that after exposure of SaOS-2 cells the steady-state-expression of ALP of BMP-2 (bone morphogenetic protein-2), as well as the expression of the SOST gene, encoding sclerostin which is considered to be an antagonist of BMP cytokines, is strongly to moderately modulated in SaOS-2 cells after exposure to Sr-polyP microparticles.7.4 Potential use for cartilage repair: Mg-polyP
Magnesium is an abundant element in the crust of the Earth and is crucially involved in mineral deposition in the body.195 An imbalance in the Mg2+ status, like hypomagnesaemia, often results in unwanted neuromuscular, cardiac or nervous disorders. Therefore, sintered, non-amorphous Ca2+-polyP was prepared by melting at 1100 °C, and tested as cartilage implants with some success.196,197 Our recent studies have not been performed with the Ca2+ salt in order to avoid and prevent Ca2+ crystal deposition in the synovial fluid, which is disadvantageous.198 We have used the Mg2+ salt formulation of polyP instead for a (potential) application in cartilage repair.115,199,200Biodegradability: no published data exist which directly showed that Mg-polyP-MP is degraded in vitro or in vivo. However, since the particles are biologically active and destroyed after incubation in the presence of ALP,71 strong indirect evidence exists that Mg-polyP-MP also undergo enzymatic hydrolysis in the presence of ALP.
Biocompatibility: until now, only cell culture experiments with human chondrocytes115,200 have been conducted; they revealed a significant upregulation of cell growth under the conditions used.
The regeneration activity has not yet been determined; animal experiments are under way.
Mechanical properties: these properties turned out to be very advantageous under ex vivo conditions. The material fabricated, especially together with hyaluronic acid,115,199,200 turned out to be very similar with respect to the strength and contractibility compared to physiological cartilage.
Morphogenetic activity: gene expression studies have been performed for collagen type 3A1 and the characteristic transcription factor SOX9 as well as for the ALP, collagen 2A1 and aggrecan.115,199,200 The steady-state-expression of these genes is significantly upregulated in the presence of Mg-polyP-MP.
Scaffold architecture: the architecture has been described. Importantly, it has been identified that the chondrocytes invade the Mg-polyP-MP-based implant material.199 This observation suggests that this polyP formulation even attracts the cells.
Manufacturing technology: the characteristics are the same as those described for the Ca-polyP-MP.
7.5 Amorphous polyP particles as drug carriers: zoledronic acid
The anionic polymer Na-polyP can be readily co-precipitated with the anionic zoledronic acid, a drug frequently used in management of metastatic bone diseases201 in the presence of Ca2+.188 During this process the mixed deposits Ca-polyP/zoledronic acid remain almost perfectly amorphous (Fig. 13E) like the Ca-polyP particles, in contrast to the deposits with pure zoledronic acid and Ca2+ which formed strong crystals (Fig. 13D). In turn, it can be postulated, and the in vitro data supported this view, that zoledronic acid encapsulated together with polyP shows a higher bio-availability. The gene expression studies revealed that the mixed amorphous particles still retain the morphogenetic activity of polyP in vitro, to induce the expression of the transcription factors SOX9 (induction of MSC towards osteoblast differentiation; Fig. 13F) and of RUNX2 (inducing chondrocyte differentiation; Fig. 13G) while simultaneously inhibiting cell growth, the zoledronic acid effect.8. Types of application
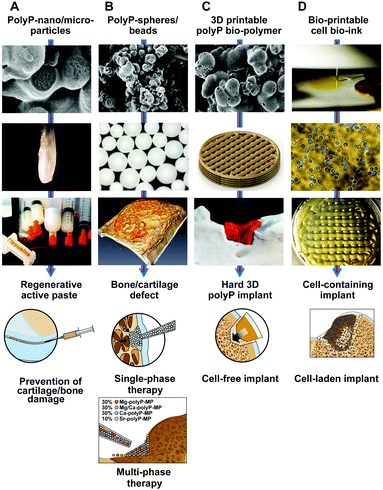
Based on the basic scientific information gathered until now, the major applied focus is put on the development of the polyP-based microparticles/nanoparticles for the prevention of osteo-articular cartilage and bone lesions and their eventual repair with implants. In particular, the cartilage and the bone tissue are characterized by a comparably low blood circulation paralleled with a low regeneration capacity of the cells. Even more, the overall percentage of cells present in bones and tissues is low and the proportion of regeneratively active cells, e.g. osteoblasts, amounts to about 4–6% of the total resident bone cells202 or chondrocytes that constitute about 2% of the total volume of articular cartilage.203 In order to exploit the properties of polyP, first that it is regeneratively active and second that it elicits metabolic energy during enzymatic hydrolysis, the polymer is not advisable to be applied as Na-polyP. As reported69 Na-polyP chelates out Ca2+ from the physiological milieu, resulting in toxic effects. This can be prevented by the fabrication of only slightly soluble salts of polyP formed with Ca2+, Sr2+ or Mg2+ from the highly soluble Na-polyP. Initially those Ca2+ salts of polyP have been sintered,204 converting the polymer into a crystalline state. In order to increase the bioavailability of the polymer and to allow a controlled enzymatic disintegration amorphous polyP salts in the form of microparticles/nanoparticles have been prepared.71 Those particles can be integrated into a paste or in bio-printed implants or processed to spheres by embedding the particles in an organic matrix like PLGA.1668.1 Regenerative active paste
A hydrogel has to be combined with polyP particles in order guarantee a sustainable fluid/gel state. A successful paste was prepared with hyaluronic acid. This anionic, nonsulfated glycosaminoglycan is an established multifunctional polymer that is used as a component in design engineered hydrogels.205 It can be used together with polyP to cross-link the two components with Mg2+.200 Also cross-linking with Ca2+ is possible; those mats have been shown to increase the steady-state-expression of the stromal cell-derived factor 1 [SDF-1α],168 a signaling molecule that acts as a strongly chemotactic chemokine.206 The paste can be readily sterilized in a syringe with ethylene oxide,207 allowing versatile application as a sealant for smaller bone defects, e.g. after removal of a bone tumor or for sinus lifting (Fig. 14).A future aspect of the developments will also include an in vivo study to elucidate whether such a polyP-hydrogel paste can be applied into the synovial fluid with the objective of learning whether this formulation can support cartilage repair, while preventing a later occurring bone defect, as anticipated.208
8.2 Single-phase polyP-based spheres
An emulsion-based embedding209 of the polyP particles into PLGA has been prepared with the formation of ≈800 μM large spheres. Those spheres have been implanted into cranial defects and were shown to undergo dissolution during a 4-week period in rats.166,191 This initial phase is accompanied by an invasion of new cells, comprising bone-mineralizing activity (Fig. 14). This biological regeneration-inducing function was found to be superior to the one caused by β-TCP controls.It is hoped that this polyP particle/sphere approach can be established as a potential single-phase therapy using distinct polyP-based spheres for a specific disorder, bone or cartilage.
8.3 Multi-phase spheres
As a consequence, the difference in the ζ potential of the polyP Ca2+, Mg2+ and Sr2+ salts of polyP (with a gradient from −50 mV [Sr2+ salt] via −42.3 mV [Ca2+ salt] to −20 mV [Mg2+ salt]; to be published) and the morphological differences of the particles (Fig. 4), varying from total spherical (the Sr2+ and the Ca2+ polymers) to more fluffy, flat aggregates (Mg2+ polymer) suggest that the particles share a different tendency to form coacervates. This difference will surely also reflect not only different release kinetics of the respective counterion from the polymeric salt but also a phase-specific differential biological effect.In turn, the polyP salts will be embedded into spheres and added to the cartilage/bone defect region as implants in a zoned arrangement. The objective of this approach would be a sequential and/or a regional repair of the damaged osteo-articular defect.
8.4 3D bio-printed implants
It is surely very correct and very scientifically based that a successful regenerative repair of a bone or cartilage region must also involve MSCs. However, here the progress can be advanced in two directions; either the cell-free implants should have the biological property to attract the MSC to the place of the defect, or, the cell-laden implants supply with the respective scaffold the MCS to the defect.At present – and for a more short-term accomplishment of the objective to provide and fabricate customized implants – the cell-free printed implants appear to be more feasible. In recent years an increasing number of studies have been published that demonstrate the rapid progress in the field of 3D printing technology (reviewed in ref. 210). In a recent study polyP in the form of inorganic, amorphous Ca2+ polyP particles was embedded into an organic poly-ε-caprolactone [PCL] matrix and printed to tissue-like scaffolds, leaving space in the potential implant for a later invasion of cells.211 The implants with their stacked architecture were determined to combine suitable biomechanical properties with polyP-based morphogenetic activity. Important to notice is again that this scaffold showed the property to attract the SaOS-2 cells most likely via the chemokine SDF-1α.
Future directions will include the optimization of the biomechanical stability of respective scaffolds in a regeneration-dependent manner. It should be attempted to fabricate a scaffold that matches the dissolution kinetics in the implanted region with the regeneration potential and hence the ingrowth efficiency of the regenerating tissue.
8.5 Cell-laden 3D bio-printed implants
Besides the potential ethical hurdles which might be anticipated, the major difficulty in the strategies is to develop suitable matrices allowing the embedding of the MCS at a lower concentration (<1 million cells per ml [g] of implant). This is a very serious difficulty, since the underlying constricting factors are complex (Fig. 14). First, the material must allow a sufficient supply of the cells with soluble nutrients/energy; then, the materials must be biocompatible and finally, the materials must allow and support proliferation and differentiation. Furthermore, both direct cell–cell contact through nurse cells212 and even extracellular vesicles affect the differentiation direction of the MSC.213 Those vesicles are abundantly present around SaOS-2 and HUVEC cells.214 In addition, it has been shown that the topology of the surrounding matrices controls the gene expression systems within MSCs and in turn also the phenotype of the differentiated cells.215 Surely a defined cocktail of growth factors will help overcome these challenges (reviewed in ref. 216); however, it is far from clear to outline which signals are sequentially expressed to direct the MSC towards the chondrogenic or osteogenic direction. Interestingly enough adenosine receptors have been implicated in the selection of those pathways;217 the related purinergic receptors have been implicated in the signaling cascade of extracellularly applied polyP.218In the studies performed by us we used Na-polyP, complexed with additional Ca2+, alginate and gelatin to stabilize a bio-ink that allowed printing of implants with bone-related SaOS-2 cells.219 The cells showed a considerable proliferation capacity, even though the reduced Young's modulus for the hydrogel, reached a value of approximately 13–14 kPa. Basically as expected and also intended, the hardness dropped during a >5 d incubation period. The cells showed distinct mineralization potency. This direction of work demonstrated that polyP as a component of the printing matrix is essential for cells to grow in the hydrogel.
8.6 Scaffold architecture with hierarchical functionalization
The ECM has a multiscale hierarchical architecture from the macro-, micro- and nanoscale (reviewed in ref. 220 and 221). It is a natural composite containing both organic components (mainly type-I collagen, but also type-III, type-IV collagen and fibrillin) and also inorganic crystalline mineral (mainly hydroxyapatite). It resembles large churches like the Cologne cathedral constructed with hybrid materials, cement and mortar. In bones the macrostructure is built by osteons, again an organized entity of osteocytes, osteoclasts, osteoblasts, canaliculi, lamellae and lacunae. The structure giving organic fibers are made of collagen fibrils (diameter of ≈ 0.5 μm) that are made of 1.5 nm fibers, enforced by nanocrystalline hydroxyapatite. These elements are made of collagen molecules, having a 2–3 nm periodicity. This hierarchical structure provides stability not only on the macroscale level but also down to the nanoscale structures. In particular, porosity is an important property providing stability to the bio-inorganic bone but also space for the infiltration of the circulation system. The porosity varies between 50 and 90%.221 Therefore, osteo-articular implants should also mimic the hierarchical structure that follows native tissues. In the ECM self-assembled collagen fibrils contribute to the major scaffold elements also in the bone, and especially in the cartilage tissue. This polymer or a related organic fibrous material has been used since the beginning as a biomimetic scaffold to fabricate hierarchically organized structures, and generating support, in bone and cartilage tissue engineering applications.222–224 Because this polymer comprises major problem as a scaffold for orthopedic tissue engineering due to the relatively poor mechanical properties,193 the fibers have been functionalized and then used for building three-dimensional porous scaffolds providing an interconnected macroporous network with pore diameters that match the spacings required for vascularization and tissue ingrowth.The physiological polymer polyP is an exceptionally suitable material to form a porous scaffold biomaterial (sketch in Fig. 15). According to the concentration of Ca2+ and the duration of exposure Na-polyP can be printed together with a hydrogel and subsequently hardened to a scaffold material useful for smaller implants.167 This mono-material (non-composite) scaffold already exhibits morphogenetic activity. In order to improve and further adapt the polyP-based scaffold to the biomechanical characteristics of bone and cartilage, organic/synthetic and organic/physiological scaffold additives have been successfully evaluated. PCL is a versatile organic/synthetic since it can be formulated with different biomechanical characteristics as well as periods for biodegradation.225 The property of PCL to be inert can be overcome with polyP, as demonstrated.211
We have applied the technique of freeze-extraction to process collagen, as an organic/physiological additive, together with polyP via a colloid formation, followed by Ca2+ crosslinking, and freeze-extraction to fabricate a macroporous hybrid biomaterial/bioscaffold material.192 The scaffold has been composed in addition to collagen with chondroitin sulfate that could be likewise linked with Ca2+. In the absence of polyP a fibrous collagen scaffold is seen that resembles the ECM (Fig. 16A and B). In the presence of polyP and after processing with Ca2+, nanoparticles of Ca-polyP are formed around the collagen fibers that measure only ≈50 nm (Fig. 16C and D). Between this hierarchically formed scaffold, fibrous matrix decorated and partially cavity-filling polyP nanoparticles, cavities/channels have been left open with a diameter of larger than 75 μm (Fig. 16C and D). The mechanical properties have been determined with a Young's modulus of 0.4 MPa. The biocompatibility of the polyP/collagen was proven both by cell proliferation experiments and with gene expression/PCR [polymerase chain reaction] studies. If this scaffold is submersed into medium plus FCS, the polyP particles fuse to extensive mat-like coacervate assemblies (Fig. 16E and F).
The suitability of the polyP matrix for bioprinting of cells within the matrix has been successfully demonstrated.219
As outlined, an interesting feature of polyP, if fabricated into particles at pH 10, the technique which has been applied for leaving the particles in an amorphous state,71 is that the particles are in a coacervation phase. This phase, according to the available data, is the form which acts also physiologically in the cell culture and surely also in vivo, where the inorganic as well as organic constituents are dissolved in a proteinaceous environment (also depicted in Fig. 15). In the future, it will be studied if already during the process of particle formation the coacervate step can be passed through.
9. Conclusion and outlook
The clue during evolution to metazoan organisms was the successful development of an insoluble matrix that was transformed to a functionally active support through an integration of enzymatic and non-enzymatic biological molecules. It is obvious that the non-cellular ECM components of the cell microenvironment play a critical role in promoting cell activity, like adhesion, spreading or migration, invasion and metastasis (reviewed in ref. 226). The interactions between the cells and their associated ECM create dynamic mechanical and metabolic energy transmitting relationships that regulate those cellular processes. A perturbation in those biophysical dynamics between cell layers and the ECM potentiates signaling pathways that regulate tumor growth, invasion, and survival.227 In the other extreme situation, a lack of any signals emanating from the ECM to the cells turns the proliferating cells to quiescent cells.228 It is the cellular AMP-activated protein kinase that functions as the energy sensor for any kind of cellular activity229 and the integrin/tensin system functions as a linker monitoring the extracellular energy status to this kinase.230 The extracellular supramolecular architecture, composed (by non-covalent linkages) of laminins, nidogens, heparan sulfate proteoglycans, perlecan, agrin and collagens, needs metabolic energy to allow the self-association of the monomeric components; they are endothermic, primarily entropy-driven phase separation processes (reviewed in ref. 231). Spontaneous complex coacervation is characterized by a negative Gibbs free energy.232As summarized in this review polyP is a physiological bioinorganic polymer undergoing coacervation after transfer of the particles (like the Ca-polyP microparticles/nanoparticles) from pH 10 to a physiological pH.
After the initial studies on the polyP metabolism in bacteria and yeasts, and then in higher organisms89,158,233 the potential applications for human hard tissue repair were proposed.64 A growth of studies occurred after the introduction of the technology to fabricate Ca-polyP microparticles.71 Subsequently, those particles had been included in scaffolds, microspheres or pastes for the application in skin,234 teeth,235 artificial blood vessels236 and for wound healing.237 In addition, due to the beneficial hardening characteristics as an implant material, a further momentum was reached, with respect to implant fabrications also in a personalized way, as well as a potential help in osteoporosis.114,167,211 A schematic time course is sketched in Fig. 17.
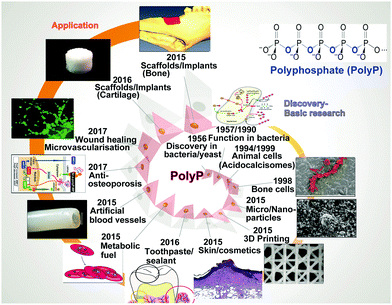 | ||
| Fig. 17 Steps in the course of the basic and applied discoveries of polyP, especially in humans. (Partially from ref. 238 [with permission].) | ||
Previously a series of naturally-derived biomacromolecules, like heparin or other polyanions, have been shown to improve the regenerative activity through their coacervation properties.239 In turn, polyP provides a first coacervate-based biomaterial that has the potential to become successfully introduced in tissue engineering (Fig. 15). It has many advantageous properties. For example, it:
– is physiologically suitable
– is amorphous
– has metabolic energy required for the maintenance of the extracellular supramolecular architecture
– provides with monomeric phosphate units as essential building groups for bone (hydroxyapatite) formation
– can be coated around (bio-inert) scaffolds
– allows the integration of biologically active components, like peptides or drugs
– is not only a scaffold supporting cellular growth but promotes growth and differentiation of cells. By this, polyP is en route to follow the formulated forward-looking concept240,241
– is not only a solid, macroscopic polymer, designed “with robustness in mind” and “manufactured by conventional engineering routes” but is provided with the potential to self-assemble at the nanoscale in a dynamic process during which new biological functionalities are gained.
With these features a new paradigm in tissue engineering might become a reality.
The new technologies, especially of the biofabrication processes applying additive manufacturing–rapid prototyping–solid freeform fabrication allowing the production of customized implants will surely revolutionize the development also in the field of osteo-articular implantology. However, there are significant challenges to overcome as outlined.242 The bone or cartilage implants can be fabricated in small dimensions – but for larger implants the central issues are suitable running systems for blood vessels, physiological supply with spatio-temporal biochemical and mechanical stimuli or the compliance/compatibility with the tissue environment. Also the manufacturing technology required for the preparation of the biomaterial has to be determined, since only then will a standardization of protocols for the fabrication and the application – from the manufacturer to the patient – become possible. Therefore, the biomaterials to be applied must be “smart”, meaning dynamic, rather than static and self-adaptable to the given tissue environment. Based on these prerequisites, skeletal stem cell biology has to be improved towards a further understanding of the signals, cytokines/chemokines and cellular interactions, directing the MSC to a functional and appropriate cell lineage. Finally the economical constraints cannot be forgotten.
The biopolymer polyP, especially being fabricated as amorphous microparticles/nanoparticles, will contribute with its described properties to develop a new generation of smart biomaterials, hopefully improving the quality of life in the aging populations worldwide.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
W. E. G. M. is a holder of an ERC Advanced Investigator Grant (No. 268476 BIOSILICA). In addition, W. E. G. M. obtained three ERC-PoC grants (Si-Bone-PoC, Grant No. 324564; MorphoVES-PoC, Grant No. 662486; and ArthroDUR, Grant No. 767234). Finally, this work was supported by a grant from the Federal Ministry for Economic Affairs and Energy (ZIM - ZF4294001 CS6), the BMBF Grant NanoOsMed (Grant No. 01DH17034A), the International Human Frontier Science Program and the BiomaTiCS research initiative of the University Medical Center, Mainz.References
- N. C. Wright, A. C. Looker, K. G. Saag, J. R. Curtis, E. S. Delzell, S. Randall and B. Dawson-Hughes, The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine, J. Bone Miner. Res., 2014, 29, 2520–2526 CrossRef PubMed.
- R. H. Carmona, Bone Health and Osteoporosis – A Report of the Surgeon General, U.S. Department of Health and Human Services, Rockville, MD, 2004 Search PubMed.
- S. Smith, Marktanalyse – Regenerative Medicine Market by Therapy (Cell Therapy, Tissue Engineering, Immunotherapy, Gene Therapy), Product (Cell-Based, Acellular), Applications (Orthopedic & Musculoskeletal Disorders, Dermatology, Oncology, Cardiology) – Forecast to 2021, MarketsandMarkets Research Private Ltd, 2017 Search PubMed.
- M. Saini, Y. Singh, P. Arora, V. Arora and K. Jain, Implant biomaterials: a comprehensive review, World J. Clin. Cases, 2015, 3, 52–57 CrossRef PubMed.
- M. E. Furth, A. Atala and M. E. Van Dyke, Smart biomaterials design for tissue engineering and regenerative medicine, Biomaterials, 2007, 28, 5068–5073 CrossRef CAS PubMed.
- A. S. Mao and D. J. Mooney, Regenerative medicine: Current therapies and future directions, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 14452–14459 CrossRef CAS PubMed.
- E. M. Tanaka and P. W. Reddien, The cellular basis for animal regeneration, Dev. Cell, 2011, 21, 172–185 CrossRef CAS PubMed.
- S. J. Forbes and N. Rosenthal, Preparing the ground for tissue regeneration: from mechanism to therapy, Nat. Med., 2014, 20, 857–869 CrossRef CAS PubMed.
- K. Cheng, F. Wu and F. Cao, Intramyocardial autologous cell engraftment in patients with ischaemic heart failure: a meta-analysis of randomised controlled trials, Heart, Lung Circ., 2013, 22, 887–894 CrossRef PubMed.
- A. Mendelson and P. S. Frenette, Hematopoietic stem cell niche maintenance during homeostasis and regeneration, Nat. Med., 2014, 20, 833–846 CrossRef CAS PubMed.
- A. Behfar, R. Crespo-Diaz, A. Terzic and B. J. Gersh, Cell therapy for cardiac repair-lessons from clinical trials, Nat. Rev. Cardiol., 2014, 11, 232–246 CrossRef PubMed.
- J. A. Buckwalter, M. J. Glimcher, R. R. Cooper and R. Recker, Bone biology. Part I: Structure, blood supply, cells, matrix, and mineralization, Instr. Course Lect., 1995, 45, 371–386 Search PubMed.
- A. Kim, H. Y. Yu, J. Heo, M. Song, J. H. Shin, J. Lim, S. J. Yoon, Y. Kim, S. Lee, S. W. Kim, W. Oh, S. J. Choi, D. M. Shin and M. S. Choo, Mesenchymal stem cells protect against the tissue fibrosis of ketamine-induced cystitis in rat bladder, Sci. Rep., 2016, 6, 30881 CrossRef CAS PubMed.
- G. A. Norambuena, M. Khoury and C. Jorgensen, Mesenchymal stem cells in osteoarticular pediatric diseases: an update, Pediatr. Res., 2012, 71, 452–458 CrossRef CAS PubMed.
- J. Freitag, D. Bates, R. Boyd, K. Shah, A. Barnard, L. Huguenin and A. Tenen, Mesenchymal stem cell therapy in the treatment of osteoarthritis: reparative pathways, safety and efficacy – a review, BMC Musculoskeletal Disord., 2016, 17, 230 CrossRef PubMed.
- C. J. Centeno, D. Busse, J. Kisiday, C. Keohan, M. Freeman and D. Karli, Increased knee cartilage volume in degenerative joint disease using percutaneously implanted, autologous mesenchymal stem cells, Pain Physician, 2008, 11, 343–353 Search PubMed.
- C. Centeno, J. Schultz and M. Cheever, Safety and complications reporting on the re-implantation of culture-expanded mesenchymal stem cells using autologous platelet lysate technique, Curr. Stem Cell Rep., 2011, 5, 81–93 CrossRef.
- P. A. Tozetti, S. R. Caruso, A. Mizukami, T. R. Fernandes, F. B. da Silva, F. Traina, D. T. Covas, M. D. Orellana and K. Swiech, Expansion strategies for human mesenchymal stromal cells culture under xeno-free conditions, Biotechnol. Prog., 2017, 33, 1358–1367 CrossRef CAS PubMed.
- F. R. Maia, A. H. Lourenço, P. L. Granja, R. M. Gonçalves and C. C. Barrias, Effect of cell density on mesenchymal stem cells aggregation in RGD-alginate 3D matrices under osteoinductive conditions, Macromol. Biosci., 2014, 14, 759–771 CrossRef CAS PubMed.
- C. C. Wyles, M. T. Houdek, A. Behfar and R. J. Sierra, Mesenchymal stem cell therapy for osteoarthritis: current perspectives, Stem Cells Cloning: Adv. Appl., 2015, 8, 117–124 Search PubMed.
- W. Zhang, R. W. Moskowitz, G. Nuki, S. Abramson, R. D. Altman, N. Arden, S. Bierma-Zeinstra, K. D. Brandt, P. Croft, M. Doherty, M. Dougados, M. Hochberg, D. J. Hunter, K. Kwoh, L. S. Lohmander and P. Tugwell, OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelines, Osteoarthritis Cartilage, 2008, 16, 137–162 CrossRef CAS PubMed.
- J. Y. Ahn, G. Park, J. S. Shim, J. W. Lee and I. H. Oh, Intramarrow injection of beta-catenin-activated, but not naive mesenchymal stromal cells stimulates self-renewal of hematopoietic stem cells in bone marrow, Exp. Mol. Med., 2010, 42, 122–131 CrossRef CAS PubMed.
- S. T. Mohanty and I. Bellantuono, Intra-femoral injection of human mesenchymal stem cells, Methods Mol. Biol., 2013, 976, 131–141 CAS.
- J. Mak, C. L. Jablonski, C. A. Leonard, J. F. Dunn, E. Raharjo, J. R. Matyas, J. Biernaskie and R. J. Krawetz, Intra-articular injection of synovial mesenchymal stem cells improves cartilage repair in a mouse injury model, Sci. Rep., 2016, 6, 23076 CrossRef CAS PubMed.
- H. K. Erben, Biomineralisation – Forschungsberichte–Biomineralization – Research Report, Schattauer, Stuttgart-New York, 1970, Preface.
- H. A. Lowenstam and S. Weiner, On Biomineralization, University Press, New York-Oxford, 1989 Search PubMed.
- L. Addadi and S. Weiner, Control and design principles in biological mineralization, Angew. Chem., Int. Ed. Engl., 1992, 31, 153–169 CrossRef.
- S. Weiner, J. Mahamid, Y. Politi, Y. Ma and L. Addadi, Overview of the amorphous precursor phase strategy in biomineralization, Front. Mater. Sci. China, 2009, 3, 104–108 CrossRef.
- S. Weiner and H. D. Wagner, The material bone: Structural-mechanical function relations, Annu. Rev. Mater. Sci., 1998, 28, 271–298 CrossRef CAS.
- G. Karsenty, H. M. Kronenberg and C. Settembre, Genetic control of bone formation, Annu. Rev. Cell Dev. Biol., 2009, 25, 629–648 CrossRef CAS PubMed.
- X. H. Wang, H. C. Schröder, M. Wiens, H. Ushijima and W. E. G. Müller, Bio-silica and bio-polyphosphate: applications in biomedicine (bone formation), Curr. Opin. Biotechnol., 2012, 23, 570–578 CrossRef CAS PubMed.
- M. Das, I. B. Sundell and P. S. Koka, Adult mesenchymal stem cells and their potency in the cell-based therapy, J. Stem Cells, 2013, 8, 1–16 Search PubMed.
- R. A. Denu, S. Nemcek, D. D. Bloom, A. D. Goodrich, J. Kim, D. F. Mosher and P. Hematti, Fibroblasts and mesenchymal stromal/stem cells are phenotypically indistinguishable, Acta Haematol., 2016, 136, 85–97 CrossRef CAS PubMed.
- A. Peters, C. Korte, D. Hesse, N. Zakharov and J. Janek, Ionic conductivity and activation energy for oxygen ion transport in superlattices – the multilayer system CSZ (ZrO2 + CaO)/Al2O3, Solid State Ionics, 2007, 178, 67–76 CrossRef CAS.
- Z. Zhu, L. M. Xue, T. Han, L. Jiao, L. P. Qin, Y. S. Li, H. C. Zheng and Q. Y. Zhang, Antiosteoporotic effects and proteomic characterization of the target and mechanism of an Er-Xian decoction on osteoblastic UMR-106 and osteoclasts induced from RAW264.7, Molecules, 2010, 15, 4695–4710 CrossRef CAS PubMed.
- W. E. G. Müller, H. C. Schröder and X. H. Wang, The understanding of the Metazoan skeletal system, based on the initial discoveries with siliceous and calcareous sponges, Mar. Drugs, 2017, 15, 172, DOI:10.3390/md15060172.
- D. O. Wagner and P. Aspenberg, Where did bone come from?, Acta Orthop., 2011, 82, 393–398 CrossRef PubMed.
- J. A. Ruben and A. A. Bennett, Evolution of bone, Evolution, 1987, 10, 1558–5646 Search PubMed.
- A. S. Posner and G. Duyckaerts, Infrared study of the carbonate in bone, teeth and francolite, Experientia, 1954, 10, 424–425 CrossRef CAS PubMed.
- A. S. Posner, Crystal chemistry of bone mineral, Physiol. Rev., 1969, 49, 760–792 CrossRef CAS PubMed.
- R. M. Biltz and E. D. Pellegrino, The nature of bone carbonate, Clin. Orthop., 1977, 129, 279–292 CAS.
- C. F. Poyart, E. Bursaux and A. Fréminet, The bone CO2 compartment: evidence for a bicarbonate pool, Respir. Physiol., 1975, 25, 89–99 CrossRef CAS PubMed.
- S. Mann, S. B. Parker, M. D. Ross, A. J. Skarnulis and R. J. Williams, The ultrastructure of the calcium carbonate balance organs of the inner ear: an ultra-high resolution electron microscopy study, Proc. R. Soc. London, Ser. B, 1983, 218, 415–424 CrossRef CAS.
- M. Pisam, C. Jammet and D. Laurent, First steps of otoliths formation of the zebrafish: role of glycogen?, Cell Tissue Res., 2002, 310, 163–168 CrossRef CAS PubMed.
- L. R. Andrade, U. Lins, M. Farina, B. Kachar and R. Thalmann, Immunogold TEM of otoconin 90 and otolin—relevance to mineralization of otoconia, and pathogenesis of benign positional vertigo, Hear. Res., 2012, 292, 14–25 CrossRef CAS PubMed.
- L. Pasteur, Mémoire sur la fermentation appelée lactique, Mém. Soc. Sci. Agric. Arts, 1857, 5, 13–37 Search PubMed.
- W. E. G. Müller, M. Wiens, T. Adell, V. Gamulin, H. C. Schröder and I. M. Müller, Bauplan of Urmetazoa: basis for genetic complexity of Metazoa, Int. Rev. Cytol., 2004, 235, 53–92 CrossRef.
- A. Krasko, V. Gamulin, J. Seack, R. Steffen, H. C. Schröder and W. E. G. Müller, Cathepsin, a major protease of the marine sponge Geodia cydonium: Purification of the enzyme and molecular cloning of cDNA, Mol. Mar. Biol. Biotechnol., 1997, 6, 296–307 CAS.
- A. Krasko, B. Lorenz, R. Batel, H. C. Schröder, I. M. Müller and W. E. G. Müller, Expression of silicatein and collagen genes in the marine sponge Suberites domuncula is controlled by silicate and myotrophin, Eur. J. Biochem., 2000, 267, 4878–4887 CrossRef CAS PubMed.
- K. Shimizu, J. Cha, G. D. Stucky and D. E. Morse, Silicatein alpha: cathepsin L-like protein in sponge biosilica, Proc. Natl. Acad. Sci. U. S. A., 1998, 95, 6234–6238 CrossRef CAS.
- J. N. Cha, K. Shimizu, Y. Zhou, S. C. Christiansen, B. F. Chmelka, G. D. Stucky and D. E. Morse, Silicatein filaments and subunits from a marine sponge direct the polymerization of silica and silicones in vitro, Proc. Natl. Acad. Sci. U. S. A., 1999, 96, 361–365 CrossRef CAS.
- W. E. G. Müller, U. Schloßmacher, X. H. Wang, A. Boreiko, D. Brandt, S. E. Wolf, W. Tremel and H. C. Schröder, Poly(silicate)-metabolizing silicatein in siliceous spicules and silicasomes of demosponges comprises dual enzymatic activities (silica-polymerase and silica-esterase), FEBS J., 2008, 275, 362–370 CrossRef PubMed.
- W. E. G. Müller, A. Krasko, B. Lorenz and H. C. Schröder, Silicatein-vermittelte Synthese von amorphen Silikaten und Siloxanen und ihre Verwendung, EP1320624 B1, 2000.
- X. H. Wang, H. C. Schröder, K. Wang, J. A. Kaandorp and W. E. G. Müller, Genetic, biological and structural hierarchies during sponge spicule formation: From soft sol-gels to solid 3D silica composite structures, Soft Matter, 2012, 8, 9501–9518 RSC.
- W. E. G. Müller, H. C. Schröder, Z. Burghard, D. Pisignano and X. H. Wang, Silicateins – A novel paradigm in bioinorganic chemistry: Enzymatic synthesis of inorganic polymeric silica, Chem. – Eur. J., 2013, 19, 5790–5804 CrossRef PubMed.
- W. E. G. Müller, X. H. Wang, V. A. Grebenjuk, M. Korzhev, M. Wiens, U. Schloßmacher and H. C. Schröder, Common genetic denominators for Ca++-based skeleton in Metazoa: role of osteoclast-stimulating factor and of carbonic anhydrase in a calcareous sponge, PLoS One, 2012, 7, e34617, DOI:10.1371/journal.pone.0034617.
- W. E. G. Müller, H. C. Schröder, U. Schlossmacher, V. A. Grebenjuk, H. Ushijima and X. H. Wang, Induction of carbonic anhydrase in SaOS-2 cells, exposed to bicarbonate and consequences for calcium phosphate crystal formation, Biomaterials, 2013, 34, 8671–8680 CrossRef PubMed.
- W. E. G. Müller, H. C. Schröder, E. Tolba, M. Neufurth, B. Diehl-Seifert and X. H. Wang, Mineralization of bone-related SaOS-2 cells under physiological hypoxic conditions, FEBS J., 2016, 283, 74–87 CrossRef PubMed.
- X. H. Wang, H. C. Schröder and W. E. G. Müller, Enzyme-based biosilica and biocalcite: biomaterials for the future in regenerative medicine, Trends Biotechnol., 2014, 32, 441–447 CrossRef CAS PubMed.
- A. S. Posner, F. Betts and N. C. Blumenthal, Properties of nucleating systems, Metab. Bone Dis. Relat. Res., 1978, 1, 179–183 CrossRef CAS.
- W. Li, W. S. Chen, P. P. Zhou, L. Cao and L. J. Yu, Influence of initial pH on the precipitation and crystal morphology of calcium carbonate induced by microbial carbonic anhydrase, Colloids Surf., B, 2013, 102, 281–287 CrossRef CAS PubMed.
- W. E. G. Müller, M. Neufurth, J. Huang, K. Wang, Q. Feng, H. C. Schröder, B. Diehl-Seifert, R. Muñoz-Espí and X. H. Wang, Non-enzymatic transformation of amorphous CaCO3 into calcium phosphate mineral after exposure to sodium phosphate in vitro: implications for in vivo hydroxyapatite bone formation, ChemBioChem, 2015, 16, 1323–1332 CrossRef PubMed.
- Y. L. Chang, C. M. Stanford and J. C. Keller, Calcium and phosphate supplementation promotes bone cell mineralization: implications for hydroxyapatite (HA)-enhanced bone formation, J. Biomed. Mater. Res., 2000, 52, 270–278 CrossRef CAS PubMed.
- G. Leyhausen, B. Lorenz, H. Zhu, W. Geurtsen, R. Bohnensack, W. E. G. Müller and H. C. Schröder, Inorganic polyphosphate in human osteoblast-like cells, J. Bone Miner. Res., 1998, 13, 803–812 CrossRef CAS PubMed.
- J. H. Morrissey, S. H. Choi and S. A. Smith, Polyphosphate: An ancient molecule that links platelets, coagulation, and inflammation, Blood, 2012, 119, 5972–5979 CrossRef CAS PubMed.
- B. Lorenz and H. C. Schröder, Mammalian intestinal alkaline phosphatase acts as highly active exopolyphosphatase, Biochim. Biophys. Acta, 2001, 1547, 254–261 CrossRef CAS.
- H. C. Schröder, L. Kurz, W. E. G. Müller and B. Lorenz, Polyphosphate in bone, Biochemistry, 2000, 65, 296–303 Search PubMed.
- Y. Hacchou, T. Uematsu, O. Ueda, Y. Usui, S. Uematsu, M. Takahashi, T. Uchihashi, Y. Kawazoe, T. Shiba, S. Kurihara, M. Yamaoka and K. Furusawa, Inorganic polyphosphate: a possible stimulant of bone formation, J. Dent. Res., 2007, 86, 893–897 CrossRef CAS PubMed.
- W. E. G. Müller, X. H. Wang, B. Diehl-Seifert, K. Kropf, U. Schloßmacher, I. Lieberwirth, G. Glasser, M. Wiens and H. C. Schröder, Inorganic polymeric phosphate/polyphosphate as an inducer of alkaline phosphatase and a modulator of intracellular Ca2+ level in osteoblasts (SaOS-2 cells) in vitro, Acta Biomater., 2011, 7, 2661–2671 CrossRef PubMed.
- K. Qiu, X. J. Zhao, C. X. Wan, C. S. Zhao and Y. W. Chen, Effect of strontium ions on the growth of ROS17/2.8 cells on porous calcium polyphosphate scaffolds, Biomaterials, 2006, 27, 1277–1286 CrossRef CAS PubMed.
- W. E. G. Müller, E. Tolba, H. C. Schröder, S. Wang, G. Glaßer, R. Muñoz-Espí, T. Link and X. H. Wang, A new polyphosphate calcium material with morphogenetic activity, Mater. Lett., 2015, 148, 163–166 CrossRef.
- L. J. Brecevic and H. Furedi-Milhofer, Precipitation of calcium phosphates from electrolyte solutions. II. The formation and transformation of precipitates, Calcif. Tissue Res., 1972, 10, 82–90 CrossRef CAS PubMed.
- M. J. Glimcher, Recent studies of the mineral phase in bone and its possible linkage to the organic matrix by protein-bound phosphate bonds, Philos. Trans. R. Soc., B, 1984, B304, 479–508 CrossRef.
- M. D. Grynpas, L. C. Bonar and M. J. Glimcher, Failure to detect an amorphous calcium-phosphate solid phase in bone mineral: a radial distribution function study, Calcif. Tissue Int., 1984, 36, 291–301 CrossRef CAS PubMed.
- L. Brecevic and A. E. Nielsen, Solubility of amorphous calcium carbonate, J. Cryst. Growth, 1989, 98, 504–510 CrossRef CAS.
- W. E. G. Müller, S. Wang, M. Neufurth, M. Kokkinopoulou, Q. Feng, H. C. Schröder and X. H. Wang, Polyphosphate as a donor of high-energy phosphate for the synthesis of ADP and ATP, J. Cell Sci., 2017, 130, 2747–2756 CrossRef PubMed.
- L. Liebermann, Nachweis der Metaphosphorsäure im Nuclein der Hefe, Pflügers Arch., 1890, 47, 155–164 CrossRef.
- A. Ascoli, Über die Plasminsäure, Hoppe-Seylers Z., 1899, 28, 437 Search PubMed.
- W. E. G. Müller, E. Tolba, H. C. Schröder and X. H. Wang, Polyphosphate: a morphogenetically active implant material serving as metabolic fuel for bone regeneration, Macromol. Biosci., 2015, 15, 1182–1197 CrossRef PubMed.
- I. S. Kulaev, V. Vagabov and T. Kulakovskaya, The Biochemistry of Inorganic Polyphosphates, John Wiley, Chichester, 2nd edn, 2004 Search PubMed.
- A. Kornberg, N. N. Rao and D. Ault-Riché, Inorganic polyphosphate: a molecule of many functions, Annu. Rev. Biochem., 1999, 68, 89–125 CrossRef CAS PubMed.
- Inorganic polyphosphates; biochemistry, biology, biotechnology, Progress in Molecular and Subcellular Biology, ed. H. C. Schröder and W. E. G. Müller, Springer Verlag, Berlin, 1999, vol. 23 Search PubMed.
- P. Langen, E. Liss and K. Lohmann, Art, Bildung und Umsatz der Polyphosphate der Hefe, in Acides ribonucléiques et polyphosphates: structure, synthèse et fonctions, Colloques internationaux du Centre National de la Recherche Scientifique No 105, Editions du Centre National de la Recherche Scientifique, Paris, 1962, pp. 604–614.
- K. Lohmann, Über das Vorkommen, und den Umsatz von Pyrophosphat in Zellen. I. Mitteilung: Nachweis und Isolierung des Pyrophosphates, Biochem. Z., 1928, 202, 466–493 CAS.
- K. Lohmann, Über das Vorkommen und den Umsatz von Pyrophosphat in Zellen. II. Mitteilung: Die Menge der leicht hydrolysierbaren P-Verbindung in tierischen und pflanzlichen Zellen, Biochem. Z., 1928, 203, 164–171 CAS.
- K. Lohmann, Über das Vorkommen und den Umsatz von Pyrophosphat in Zellen. III. Mitteilung: Das physiologische Verhalten des Pyrophosphats, Biochem. Z., 1928, 203, 172–207 CAS.
- H. Holzer and F. Lynen, Über den aeroben Phosphatbedarf der Hefe. III. Labil an die Struktur gebundenes Phosphat in lebender Hefe, Liebigs Ann. Chem., 1950, 569, 138–148 CrossRef CAS.
- I. S. Kulaev and A. N. Belozerskij, Electrophoretic studies on polyphosphate-ribonucleic acid complexes from Aspergillus niger, Proc. Acad. Sci. USSR, 1958, 120, 128–131 Search PubMed.
- P. Langen and E. Liss, Differenzierung des Orthophosphates der Hefezelle, Biochem. Z., 1960, 392, 403–406 Search PubMed.
- A. Kornberg, S. R. Kornberg and E. S. Simms, Metaphosphate synthesis by an enzyme from Escherichia coli, Biochim. Biophys. Acta, 1956, 26, 215–227 CrossRef.
- S. R. Kornberg, Adenosine triphosphate synthesis from polyphosphates by an enzyme from Escherichia coli, Biochim. Biophys. Acta, 1957, 26, 294–300 CrossRef CAS.
- A. Kornberg, Inorganic polyphosphate: toward making a forgottenpolymer unforgettable, J. Bacteriol., 1995, 177, 491–496 CrossRef CAS PubMed.
- N. N. Rao, M. R. Gomez-Garcia and A. Kornberg, Inorganic polyphosphate: essential for growth and survival, Annu. Rev. Biochem., 2009, 78, 605–647 CrossRef CAS PubMed.
- B. Lorenz, W. E. G. Müller, I. S. Kulaev and H. C. Schröder, Purification and characterization of an exopolyphosphatase activity from Saccharomyces cerevisiae, J. Biol. Chem., 1994, 269, 22198–22204 CAS.
- S. R. Pallerla, S. Knebel, T. Polen, P. Klauth, J. Hollender, V. F. Wendisch and S. M. Schoberth, Formation of volutin granules in Corynebacterium glutamicum, FEMS Microbiol. Lett., 2005, 243, 133–140 CrossRef CAS PubMed.
- R. Docampo, W. de Souza, K. Miranda, P. Rohloff and S. N. Moreno, Acidocalcisomes – conserved from bacteria to man, Nat. Rev. Microbiol., 2005, 3, 251–261 CrossRef CAS PubMed.
- M. Szumera, Structural investigations of silicate-phosphate glasses containing MoO3 by FTIR, Raman and 31P MAS NMR spectroscopies, Spectrochim. Acta, Part A, 2014, 130, 1–6 CrossRef CAS PubMed.
- H. Zhang, M. R. Gómez-García, X. Shi, N. N. Rao and A. Kornberg, Polyphosphate kinase 1, a conserved bacterial enzyme, in a eukaryote, Dictyostelium discoideum, with a role in cytokinesis, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 16486–16491 CrossRef CAS PubMed.
- D. Secco, C. Wang, H. Shou and J. Whelan, Phosphate homeostasis in the yeast Saccharomyces cerevisiae, the key role of the SPX domain-containing proteins, FEBS Lett., 2012, 586, 289–295 CrossRef CAS PubMed.
- K. Ohashi, S. Kawai and K. Murata, Identification and characterization of a human mitochondrial NAD kinase, Nat. Commun., 2012, 3, 1248 CrossRef PubMed.
- C. Azevedo, T. Livermore and A. Saiardi, Protein polyphosphorylation of lysine residues by inorganic polyphosphate, Mol. Cell, 2015, 58, 71–82 CrossRef CAS PubMed.
- R. Fortuna, H. C. Anderson, R. Carty and S. W. Sajdera, Enzymatic characterization of the chondrocytic alkaline phosphatase isolated from bovine fetal epiphyseal cartilage, Biochim. Biophys. Acta, 1979, 570, 291–302 CrossRef CAS.
- M. Tammenkoski, K. Koivula, E. Cusanelli, M. Zollo, C. Steegborn, A. A. Baykov and R. Lahti, Human metastasis regulator protein H-prune is a short-chain exopolyphosphatase, Biochemistry, 2008, 47, 9707–9713 CrossRef CAS PubMed.
- B. Lorenz, J. Leuck, D. Köhl, W. E. G. Müller and H. C. Schröder, Anti-HIV-1 activity of inorganic polyphosphates, J. Acquired Immune Defic. Syndr. Hum. Retrovirol., 1997, 14, 110–118 CrossRef CAS.
- F. A. Ruiz, C. R. Lea, E. Oldfield and R. Docampo, Human platelet dense granules contain polyphosphate and are similar to acidocalcisomes of bacteria and unicellular eukaryotes, J. Biol. Chem., 2004, 279, 44250–44257 CrossRef CAS PubMed.
- R. L. Ryall, The future of stone research: rummagings in the attic, Randall's plaque, nanobacteria, and lessons from phylogeny, Urol. Res., 2008, 36, 77–97 CrossRef PubMed.
- A. Chrissanthopoulos, P. Malkaj and E. Dalas, Calcium phosphate crystallization on polyglycine, polytyrosine and polymethionine, Mater. Lett., 2006, 60, 3874–3878 CrossRef CAS.
- R. S. Lee, M. V. Kayser and S. Y. Ali, Calcium phosphate microcrystal deposition in the human intervertebral disc, J. Anat., 2006, 208, 13–19 CrossRef CAS PubMed.
- J. W. Bijlsma and K. Knahr, Strategies for the prevention and management of osteoarthritis of the hip and knee, Best Pract. Res., Clin. Rheumatol., 2007, 21, 59–76 CrossRef CAS PubMed.
- M. D. Ferrer, M. M. Pérez, M. M. Cànaves, J. M. Buades, C. Salcedo and J. Perelló, A novel pharmacodynamic assay to evaluate the effects of crystallization inhibitors on calcium phosphate crystallization in human plasma, Sci. Rep., 2017, 7, 6858 CrossRef CAS PubMed.
- A. Sánchez-Navas, A. Martín-Algarra, M. Sánchez-Román, C. Jiménez-López, F. Nieto and A. Ruiz-Bustos, Crystal growth of inorganic and biomediated carbonates and phosphates, in Adv. Top. Cryst. Growth, ed. S. O. Ferreira, InTech, 2013 DOI:10.5772/52062.
- A. Momeni and M. J. Filiaggi, Degradation and hemostatic properties of polyphosphate coacervates, Acta Biomater., 2016, 41, 328–341 CrossRef CAS PubMed.
- C. Schmitt and S. L. Turgeon, Protein/polysaccharide complexes and coacervates in food systems, Adv. Colloid Interface Sci., 2011, 167, 63–70 CrossRef CAS PubMed.
- W. E. G. Müller, E. Tolba, M. Ackermann, M. Neufurth, S. Wang, Q. Feng, H. C. Schröder and X. H. Wang, Fabrication of amorphous strontium polyphosphate microparticles that induce mineralization of bone cells in vitro and in vivo, Acta Biomater., 2017, 50, 89–101 CrossRef PubMed.
- W. E. G. Müller, M. Ackermann, E. Tolba, M. Neufurth, S. Wang, H. C. Schröder and X. H. Wang, A bio-imitating approach to fabricate an artificial matrix for cartilage tissue engineering using magnesium-polyphosphate and hyaluronic acid, RSC Adv., 2016, 6, 88559–88570 RSC.
- R. Docampo, Acidocalcisomes and polyphosphate granules, in Inclusions in Prokaryotes, Microbiol. Monogr., ed. J. M. Shively, Springer-Verlag, Berlin Heidelberg, 2006, pp. 53–70 Search PubMed.
- A. O. McIntosh and W. L. Jablonski, X-Ray diffraction powder patterns of calcium phosphates, Anal. Chem., 1956, 28, 1424–1427 CrossRef CAS.
- F. Granados, J. Bonifacio and J. Serrano, Synthesis and characterization of calcium phosphate and its relation to Cr(VI) adsorption properties, Rev. Int. Contam. Ambiental, 2010, 26, 129–134 Search PubMed.
- A. C. Tas, Submicron spheres of amorphous calcium phosphate forming in a stirred SBF solution at 55 °C, J. Non-Cryst. Solids, 2014, 400, 27–32 CrossRef.
- A. C. Tas, The use of physiological solutions or media in calcium phosphate synthesis and processing, Acta Biomater., 2014, 10, 1771–1792 CrossRef CAS PubMed.
- B. N. Bachra and O. R. Trautz, Carbonic anhydrase and the precipitation of apatite, Science, 1962, 137, 337–338 CAS.
- B. N. Bachra, O. R. Trautz and S. L. Simon, Precipitation of calcium carbonates and phosphates. I. spontaneous precipitation of calcium carbonates and phosphates under physiological conditions, Arch. Biochem. Biophys., 1963, 103, 124–138 CrossRef CAS PubMed.
- B. N. Bachra, Precipitation of calcium carbonates and phosphates from metastable solutions, Ann. N. Y. Acad. Sci., 1963, 109, 251–255 CrossRef PubMed.
- B. N. Bachra, O. R. Trautz and S. L. Simon, Precipitation of calcium carbonates and phosphates. III. the effect of magnesium and fluoride ions on the spontaneous precipitation of calcium carbonates and phosphates, Arch. Oral Biol., 1965, 10, 731–738 CrossRef CAS PubMed.
- B. N. Bachra, A. E. Sobel and J. W. Stanford, Calcification. XXIV. mineralization of collagen and other fibers, Arch. Biochem. Biophys., 1959, 84, 79–95 CrossRef CAS PubMed.
- B. N. Bachra and A. E. Sobel, Calcification. XXV. mineralization of reconstituted collagen, Arch. Biochem. Biophys., 1959, 85, 9–18 CrossRef CAS PubMed.
- I. C. Sanchez, Entropy of living versus non-living systems, J. Mod. Phys., 2011, 2, 654–657 CrossRef CAS.
- H. Ge and H. Qian, Physical origins of entropy production, free energy dissipation, and their mathematical representations, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2010, 81, 051133 CrossRef PubMed.
- F. Lippman, Metabolic generation and utilization of phosphate bond energy, Adv. Enzymol., 1941, 1, 99–162 Search PubMed.
- V. S. Tagliabracci, J. L. Engel, J. Wen, S. E. Wiley, C. A. Worby, L. N. Kinch, J. Xiao, N. V. Grishin and J. E. Dixon, Secreted kinase phosphorylates extracellular proteins that regulate biomineralization, Science, 2012, 336, 1150–1153 CrossRef CAS PubMed.
- V. S. Tagliabracci, S. E. Wiley, X. Guo, L. N. Kinch, E. Durrant, J. Wen, J. Xiao, J. Cui, K. B. Nguyen, J. L. Engel, J. J. Coon, N. Grishin, L. A. Pinna, D. J. Pagliarini and J. E. Dixon, A single kinase generates the majority of the secreted phosphoproteome, Cell, 2015, 161, 1619–1632 CrossRef CAS PubMed.
- M. R. Bordoli, J. Yum, S. B. Breitkopf, J. N. Thon, J. E. Italiano, Jr., J. Xiao, C. Worby, S. K. Wong, G. Lin, M. Edenius, T. L. Keller, J. M. Asara, J. E. Dixon, C. Y. Yeo and M. Whitman, A secreted tyrosine kinase acts in the extracellular environment, Cell, 2014, 158, 1033–1044 CrossRef CAS PubMed.
- Z. Zheng, A. Goncearenco and I. N. Berezovsky, Nucleotide binding database NBDB – a collection of sequence motifs with specific protein–ligand interactions, Nucleic Acids Res., 2016, 44, D301–D307 CrossRef CAS PubMed.
- P. Narayan, A. Orte, R. W. Clarke, B. Bolognesi, S. Hook, K. A. Ganzinger, S. Meehan, M. R. Wilson, C. M. Dobson and D. Klenerman, The extracellular chaperone clusterin sequesters oligomeric forms of the amyloid-β(1-40) peptide, Nat. Struct. Mol. Biol., 2011, 19, 79–83 Search PubMed.
- J. K. Tsuruta, K. Wong, I. B. Fritz and M. D. Griswold, Structural analysis of sulphated glycoprotein 2 from amino acid sequence. Relationship to clusterin and serum protein 40,40, Biochem. J., 1990, 268, 571–578 CrossRef CAS PubMed.
- F. Di Virgilio and E. Adinolfi, Extracellular purines, purinergic receptors and tumor growth, Oncogene, 2017, 36, 293–303 CrossRef CAS PubMed.
- A. V. Birk, M. J. Broekman, E. M. Gladek, H. D. Robertson, J. H. Drosopoulos, A. J. Marcus and H. H. Szeto, Role of extracellular ATP metabolism in regulation of platelet reactivity, J. Lab. Clin. Med., 2002, 140, 166–175 CrossRef CAS PubMed.
- F. A. Redegeld, P. Smith, S. Apasov and M. V. Sitkovsky, Phosphorylation of T-lymphocyte plasma membrane-associated proteins by ectoprotein kinases: implications for a possible role for ectophosphorylation in T-cell effector functions, Biochim. Biophys. Acta, 1997, 1328, 151–165 CrossRef CAS.
- J. Zeck, J. Schallheim, S. Q. Lew and L. DePalma, Whole blood platelet aggregation and release reaction testing in uremic patients, BioMed Res. Int., 2013, 486290 Search PubMed.
- W. E. G. Müller, E. Tolba, Q. Feng, H. C. Schröder, J. S. Markl, M. Kokkinopoulou and X. H. Wang, Amorphous Ca2+ polyphosphate nanoparticles regulate ATP level in bone-like SaOS-2 cells, J. Cell Sci., 2015, 128, 2202–2207 CrossRef PubMed.
- W. E. G. Müller, D. Relkovic, M. Ackermann, S. F. Wang, M. Neufurth, A. Paravic-Radicevic, H. Ushijima, H. C. Schröder and X. H. Wang, Enhancement of wound healing in normal and diabetic mice by topical application of amorphous polyphosphate – superior effect of the host-guest composite material composed of collagen (host) and polyphosphate (guest), Polymers, 2017, 9, 300, DOI:10.3390/polym9070300.
- P. Dzeja and A. Terzic, Adenylate kinase and AMP signaling networks: metabolic monitoring, signal communication and body energy sensing, Int. J. Mol. Sci., 2009, 10, 1729–1772 CrossRef CAS PubMed.
- H.-J. Choo, B.-W. Kim, O.-B. Kwon, C. S. Lee, J.-S. Choi and Y.-G. Ko, Secretion of adenylate kinase 1 is required for extracellular ATP synthesis in C2C12 myotubes, Exp. Mol. Med., 2008, 40, 220–228 CrossRef CAS PubMed.
- E. E. Quillen, G. C. Haslam, H. S. Samra, D. Amani-Taleshi, J. A. Knight, D. E. Wyatt, S. C. Bishop, K. K. Colvert, M. L. Richter and P. A. Kitos, Ectoadenylate kinase and plasma membrane ATP synthase activities of human vascular endothelial cells, J. Biol. Chem., 2006, 281, 20728–20737 CrossRef CAS PubMed.
- E. Pavlov, R. Aschar-Sobbi, M. Campanella, R. J. Turner, M. R. Gómez-García and A. Y. Abramov, Inorganic polyphosphate and energy metabolism in mammalian cells, J. Biol. Chem., 2010, 285, 9420–9428 CrossRef CAS PubMed.
- H. A. Krebs, Some aspects of the energy transformation in living matter, Br. Med. Bull., 1953, 9, 97–104 CrossRef CAS PubMed.
- H. Yan and M. D. Tsai, Nucleoside monophosphate kinases: structure, mechanism, and substrate specificity, Adv. Enzymol. Relat. Areas Mol. Biol., 1999, 73, 103–134 CAS.
- I. R. Orriss, M. L. Key, M. O. R. Hajjawi and T. R. Arnett, Extracellular ATP released by osteoblasts is a key local inhibitor of bone mineralization, PLoS One, 2013, 8, e69057 CAS.
- V. Sivaramakrishnan and S. J. Fountain, Evidence for extracellular ATP as a stress signal in a single-celled organism, Eukaryotic Cell, 2015, 14, 775–782 CrossRef CAS PubMed.
- A. Brandao-Burch, M. L. Key, J. J. Patel, T. R. Arnett and I. R. Orriss, The P2X7 receptor is an important regulator of extracellular ATP levels, Front. Endocrinol., 2012, 3, 41 Search PubMed.
- R. Altmann, Die Elementarorganismen und ihre Beziehungen zu den Zellen, Veith, Leipzig, 1890 Search PubMed.
- M. Klingenberg, The ADP and ATP transport in mitochondria and its carrier, Biochim. Biophys. Acta, 2008, 1778, 1978–2021 CrossRef CAS PubMed.
- F. I. Ataullakhanov and V. M. Vitvitsky, What determines the intracellular ATP concentration, Biosci. Rep., 2002, 22, 501–511 CrossRef CAS PubMed.
- E. M. Schwiebert and A. Zsembery, Extracellular ATP as a signaling molecule for epithelial cells, Biochim. Biophys. Acta, 2003, 1615, 7–32 CrossRef CAS.
- D. S. Miller and S. B. Horowitz, Intracellular compartmentalization of adenosine triphosphate, J. Biol. Chem., 1986, 261, 13911–13915 CAS.
- T. Miyazaki, M. Iwasawa, T. Nakashima, S. Mori, K. Shigemoto, H. Nakamura, H. Katagiri, H. Takayanagi and S. Tanaka, Intracellular and extracellular ATP coordinately regulate the inverse correlation between osteoclast survival and bone resorption, J. Biol. Chem., 2012, 287, 37808–37823 CrossRef CAS PubMed.
- X. H. Wang, H. C. Schröder and W. E. G. Müller, Polyphosphate as a metabolic fuel in Metazoa: A foundational breakthrough invention for biomedical applications, Biotechnol. J., 2016, 11, 11–30 CrossRef CAS PubMed.
- K. D. Kumble and A. Kornberg, Inorganic polyphosphate in mammalian cells and tissues, J. Biol. Chem., 1995, 270, 5818–5822 CrossRef CAS PubMed.
- F. Müller, N. J. Mutch, W. A. Schenk, S. A. Smith, L. Esterl, H. M. Spronk, S. Schmidbauer, W. A. Gahl, J. H. Morrissey and T. Renné, Platelet polyphosphates are proinflammatory and procoagulant mediators in vivo, Cell, 2009, 139, 1143–1156 CrossRef PubMed.
- R. Stoop, Smart biomaterials for tissue engineering of cartilage, Injury, 2008, 39(suppl 1), S77–S87 CrossRef PubMed.
- M. Gawaz and S. Vogel, Platelets in tissue repair: control of apoptosis and interactions with regenerative cells, Blood, 2013, 122, 2550–2554 CrossRef CAS PubMed.
- A. Malhotra, M. Pelletier, R. Oliver, C. Christou and W. R. Walsh, Platelet-rich plasma and bone defect healing, Tissue Eng., Part A, 2014, 20, 2614–2633 CrossRef CAS PubMed.
- L. Kuru, G. S. Griffiths, A. Petrie and I. Olsen, Alkaline phosphatase activity is upregulated in regenerating human periodontal cells, J. Periodontal Res., 1999, 34, 123–127 CrossRef CAS PubMed.
- X. B. Zeng, H. Hu, L. Q. Xie, F. Lan, W. Jiang, Y. Wu and Z. W. Gu, Magnetic responsive hydroxyapatite composite scaffolds construction for bone defect reparation, Int. J. Nanomed., 2012, 7, 3365–3378 CrossRef CAS PubMed.
- M. Tallawi, E. Rosellini, N. Barbani, M. G. Cascone, R. Rai, G. Saint-Pierre and A. R. Boccaccini, Strategies for the chemical and biological functionalization of scaffolds for cardiac tissue engineering: a review, J. R. Soc., Interface, 2015, 12, 20150254 CrossRef PubMed.
- X. H. Wang, M. Ackermann, S. Wang, E. Tolba, M. Neufurth, Q. Feng, H. C. Schröder and W. E. G. Müller, Amorphous polyphosphate/amorphous calcium carbonate implant material with enhanced bone healing efficacy in a critical-size-defect in rats, Biomed. Mater., 2016, 11, 035005 CrossRef PubMed.
- W. E. G. Müller, E. Tolba, H. C. Schröder, M. Neufurth, S. Wang, T. Link, B. Al-Nawas and X. H. Wang, A new printable and durable N,O-carboxymethyl chitosan-Ca2+-polyphosphate complex with morphogenetic activity, J. Mater. Chem. B, 2015, 3, 1722–1730 RSC.
- M. Ackermann, X. H. Wang, S. WangF, M. Neufurth, H. C. Schröder, F. E. Isemer and W. E. G. Müller, Collagen-inducing biologization of prosthetic material for hernia repair: polypropylene meshes coated with polyP/collagen, J. Biomed. Mater. Res., Part B, 2017 DOI:10.1002/jbm.b.34016.
- W. E. G. Müller, X. H. Wang, P. Proksch, C. C. Perry, R. Osinga, J. Gardères and H. C. Schröder, Principles of biofouling protection in marine sponges: a model for the design of novel biomimetic and bio-inspired coatings in the marine environment?, Mar. Biotechnol., 2013, 15, 375–398 CrossRef PubMed.
- M. Nakamura, N. Hori, H. Ando, S. Namba, T. Toyama, N. Nishimiya and K. Yamashita, Surface free energy predominates in cell adhesion to hydroxyapatite through wettability, Mater. Sci. Eng., C, 2016, 62, 283–292 CrossRef CAS PubMed.
- T. Albrektsson and C. Johansson, Osteoinduction, osteoconduction and osseointegration, Eur. Spine J., 2001, 10(suppl 2), S96–S101 Search PubMed.
- R. Smeets, A. Kolk, M. Gerressen, O. Driemel, O. Maciejewski, B. Hermanns-Sachweh, D. Riediger and J. M. Stein, A new biphasic osteoinductive calcium composite material with a negative Zeta potential for bone augmentation, Head Face Med., 2009, 5, 13 Search PubMed.
- S. H. Choi, W. S. Jeong, J. Y. Cha, J. H. Lee, K. J. Lee, H. S. Yu, E. H. Choi, K. M. Kim and C. J. Hwang, Overcoming the biological aging of titanium using a wet storage method after ultraviolet treatment, Sci. Rep., 2017, 7, 3833 CrossRef PubMed.
- L. A. Buchanan and A. El-Ghannam, Effect of bioactive glass crystallization on the conformation and bioactivity of adsorbed proteins, J. Biomed. Mater. Res., Part A, 2010, 93, 537–546 Search PubMed.
- I. Ostolska and M. Wiśniewska, Application of the zeta potential measurements to explanation of colloidal Cr2O3 stability mechanism in the presence of the ionic polyamino acids, Colloid Polym. Sci., 2014, 292, 2453–2464 CAS.
- D. F. Franco, H. S. Barud, S. Santagneli, R. S. Lamarca, B. F. Santos, M. A. P. Silva, L. F. C. de Oliveira, S. J. L. Ribeiro and M. Nalin, Preparation and structural characterization of sodium polyphosphate coacervate as a precursor for optical materials, Mater. Chem. Phys., 2016, 180, 114–121 CrossRef CAS.
- J. S. Bae, W. Lee and A. R. Rezaie, Polyphosphate elicits pro-inflammatory responses that are counteracted by activated protein C in both cellular and animal models, J. Thromb. Haemostasis, 2012, 10, 1145–1151 CrossRef CAS PubMed.
- X. H. Wang, J. Huang, K. Wang, M. Neufurth, H. C. Schröder, S. Wang and W. E. G. Müller, The morphogenetically active polymer, inorganic polyphosphate complexed with GdCl3, as an inducer of hydroxyapatite formation in vitro, Biochem. Pharmacol., 2016, 102, 97–106 CrossRef CAS PubMed.
- H. J. Hausser and R. E. Brenner, Phenotypic instability of SaOS-2 cells in long-term culture, Biochem. Biophys. Res. Commun., 2005, 333, 216–222 CrossRef CAS PubMed.
- M. Wiens, X. H. Wang, H. C. Schröder, U. Kolb, U. Schlossmacher, H. Ushijima and W. E. G. Müller, The role of biosilica in the osteoprotegerin/RANKL ratio in human osteoblast-like cells, Biomaterials, 2010, 31, 7716–7725 CrossRef CAS PubMed.
- C. A. Gregory, W. G. Gunn, A. Peister and D. J. Prockop, An Alizarin red-based assay of mineralization by adherent cells in culture: comparison with cetylpyridinium chloride extraction, Anal. Biochem., 2004, 329, 77–84 CrossRef CAS PubMed.
- X. H. Wang, M. Ackermann, M. Neufurth, S. Wang, H. C. Schröder and W. E. G. Müller, Morphogenetically-active barrier membrane for guided bone regeneration, based on amorphous polyphosphate, Mar. Drugs, 2017, 15, 142, DOI:10.3390/md15050142.
- X. Zhang, W. G. Guo, H. L. Cui, H. Y. Liu, Y. Zhang, W. E. G. Müller and F. Z. Cui, In vitro and in vivo enhancement of osteogenic capacity in a synthetic BMP-2 derived peptide-coated mineralized collagen composite, J. Tissue Eng. Regener. Med., 2016, 10, 99–107 CrossRef CAS PubMed.
- Q. Lv, K. Hu, Q. Feng and F. Cui, Preparation of insoluble fibroin/collagen films without methanol treatment and the increase of its flexibility and cytocompatibility, J. Appl. Polym. Sci., 2008, 109, 1577–1584 CrossRef CAS.
- T. M. L. Knothe, T. D. Falls, S. H. McBride, R. Atit and U. R. Knothe, Mechanical modulation of osteochondroprogenitor cell fate, Int. J. Biochem. Cell Biol., 2008, 40, 2720–2738 CrossRef PubMed.
- J. Gao, X. L. Yan, R. Li, Y. Liu, W. He, S. Sun, Y. Zhang, B. Liu, J. Xiong and N. Mao, Characterization of OP9 as authentic mesenchymal stem cell line, J. Genet. Genomics, 2010, 37, 475–482 CrossRef CAS PubMed.
- A. Nazempour and B. J. Van Wie, Chondrocytes, mesenchymal stem cells, and their combination in articular cartilage regenerative medicine, Ann. Biomed. Eng., 2016, 44, 1325–1354 CrossRef CAS PubMed.
- W. E. G. Müller, M. Neufurth, S. Wang, M. Ackermann, R. Muñoz-Espí, Q. Feng, Q. Lu, H. C. Schröder and X. H. Wang, Amorphous, smart, and bioinspired polyphosphate nano/microparticles: a biomaterial for regeneration and repair of osteo-articular impairments in situ, Int. J. Mol. Sci., 2018, 19, 427 CrossRef PubMed.
- H. Orimo, The mechanism of mineralization and the role of alkaline phosphatase in health and disease, J. Nippon Med. Sch., 2010, 77, 4–12 CrossRef CAS PubMed.
- S. M. Oliveira, R. A. Ringshia, R. Z. Legeros, E. Clark, M. J. Yost, L. Terracio and C. C. Teixeira, An improved collagen scaffold for skeletal regeneration, J. Biomed. Mater. Res., Part A, 2010, 94, 371–379 Search PubMed.
- X. H. Wang, S. Wang, F. He, E. Tolba, H. C. Schröder, B. Diehl-Seifert and W. E. G. Müller, Polyphosphate as a bioactive and biodegradable implant material: induction of bone regeneration in rats, Adv. Eng. Mater., 2016, 18, 1406–1417 CrossRef CAS.
- W. E. G. Müller, M. Neufurth, M. Ackermann, E. Tolba, S. Wang, Q. Feng, H. C. Schröder and X. H. Wang, Fabrication of a new physiological macroporous hybrid biomaterial/bioscaffold material based on polyphosphate and collagen by freeze-extraction, J. Mater. Chem. B, 2017, 5, 3823–3835 RSC.
- F. J. O'Brien, Biomaterials & scaffolds for tissue engineering, Mater. Today, 2011, 14, 88–95 CrossRef.
- W. E. G. Müller, X. H. Wang and H. C. Schröder, New target sites for treatment of osteoporosis, in Blue Biotechnology, Progress in Molecular and Subcellular Biology, ed. W. E. G. Müller, X. H. Wang and H. C. Schröder, 2017, vol. 55, pp. 187–219 Search PubMed.
- W. Jahnen-Dechent and M. Ketteler, Magnesium basics, Clin. Kidney J., 2012, 5(suppl 1), i3–i14 CrossRef CAS PubMed.
- R. M. Pilliar, M. J. Filiaggi, J. D. Wells, M. D. Grynpas and R. A. Kandel, Porous calcium polyphosphate scaffolds for bone substitute applications – in vitro characterization, Biomaterials, 2001, 22, 963–972 CrossRef CAS PubMed.
- R. A. Kandel, M. Grynpas, R. Pilliar, J. Lee, J. Wang, S. Waldman, P. Zalzal and M. Hurtig, Repair of osteochondral defects with biphasic cartilage-calcium polyphosphate constructs in a sheep model, Biomaterials, 2006, 27, 4120–4131 CrossRef CAS PubMed.
- J. L. Rosales-Alexander, J. Balsalobre Aznar and C. Magro-Checa, Calcium pyrophosphate crystal deposition disease: diagnosis and treatment, Open Access Rheumatol.: Res. Rev., 2014, 6, 39–47 CrossRef CAS PubMed.
- W. E. G. Müller, M. Neufurth, S. Wang, E. Tolba, H. C. Schröder and X. H. Wang, Morphogenetically active scaffold for osteochondral repair (polyphosphate/alginate/N,O-carboxymethyl chitosan), Eur. Cells Mater., 2016, 31, 174–190 CrossRef.
- X. H. Wang, M. Ackermann, E. Tolba, M. Neufurth, F. Wurm, Q. Feng, S. Wang, H. C. Schröder and W. E. G. Müller, Artificial cartilage bio-matrix formed of hyaluronic acid and Mg2+-polyphosphate, Eur. Cells Mater., 2016, 32, 271–283 CrossRef CAS PubMed.
- T. J. Polascik and V. Mouraviev, Zoledronic acid in the management of metastatic bone disease, Ther. Clin. Risk Manage., 2008, 4, 261–268 CrossRef CAS.
- R. Florencio-Silva, G. R. Sasso, E. Sasso-Cerri, M. J. Simões and P. S. Cerri, Biology of bone tissue: Structure, function, and factors that influence bone cells, BioMed Res. Int., 2015, 421746 Search PubMed.
- S. A. J. Fox, A. Bedi and S. A. Rodeo, The basic science of articular cartilage: structure, composition, and function, Sports Health, 2009, 1, 461–468 CrossRef PubMed.
- Y. L. Ding, Y. W. Chen, Y. J. Qin, G. Q. Shi, X. X. Yu and C. X. Wan, Effect of polymerization degree of calcium polyphosphate on its microstructure and in vitro degradation performance, J. Mater. Sci.: Mater. Med., 2008, 19, 1291–1295 CrossRef CAS PubMed.
- C. B. Highley, G. D. Prestwich and J. A. Burdick, Recent advances in hyaluronic acid hydrogels for biomedical applications, Curr. Opin. Biotechnol., 2016, 40, 35–40 CrossRef CAS PubMed.
- R. P. Cherla and R. K. Ganju, Stromal cell-derived factor 1 alpha-induced chemotaxis in T cells is mediated by nitric oxide signaling pathways, J. Immunol., 2001, 166, 3067–3074 CrossRef CAS.
- J. N. Kearney, U. C. Franklin, V. Aguirregoicoa and K. T. Holland, Evaluation of ethylene oxide sterilization of tissue implants, J. Hosp. Infect., 1989, 13, 71–80 CrossRef CAS PubMed.
- A. H. Gomoll, H. Madry, G. Knutsen, N. van Dijk, R. Seil, M. Brittberg and E. Kon, The subchondral bone in articular cartilage repair: current problems in the surgical management, Knee Surg. Sports Traumatol. Arthrosc., 2010, 18, 434–447 CrossRef PubMed.
- F. Gabler, S. Frauenschuh, J. Ringe, C. Brochhausen, P. Götz, C. J. Kirkpatrick, M. Sittinger, H. Schubert and R. Zehbe, Emulsion-based synthesis of PLGA-microspheres for the in vitro expansion of porcine chondrocytes, Biomol. Eng., 2007, 24, 515–520 CrossRef CAS PubMed.
- H. Cui, M. Nowicki, J. P. Fisher and L. G. Zhang, 3D bioprinting for organ regeneration, Adv. Healthcare Mater., 2017, 6, 1601118 CrossRef PubMed.
- M. Neufurth, X. H. Wang, S. F. Wang, R. Steffen, M. Ackermann, N. D. Haep, H. C. Schröder and W. E. G. Müller, 3D printing of hybrid (bio)materials for bone tissue engineering: Calcium-polyphosphate microparticles encapsulated by polycaprolactone, Acta Biomater., 2017, 64, 377–388 CrossRef CAS PubMed.
- S. Chadi, J. Polyte, L. Lefevre, J. Castille, A. Ehanno, J. Laubier, F. Jaffrézic and F. Le Provost, Phenotypic and molecular alterations in the mammary tissue of R-spondin1 knock-out mice during pregnancy, PLoS One, 2016, 11, e0162566 Search PubMed.
- E. Crompot, M. Van Damme, K. Pieters, M. Vermeersch, D. Perez-Morga, P. Mineur, M. Maerevoet, N. Meuleman, D. Bron, L. Lagneaux and B. Stamatopoulos, Extracellular vesicles of bone marrow stromal cells rescue chronic lymphocytic leukemia B cells from apoptosis, enhance their migration and induce gene expression modifications, Haematologica, 2017, 102, 1594–1604 CrossRef PubMed.
- W. E. G. Müller, S. Wang, M. Wiens, M. Neufurth, M. Ackermann, D. Relkovic, M. Kokkinopoulou, Q. Feng, H. C. Schröder and X. H. Wang, Uptake of polyphosphate microparticles in vitro (SaOS-2 and HUVEC cells) followed by an increase of the intracellular ATP pool size, PLoS One, 2017, 12, e0188977 Search PubMed.
- G. Li, Y. Song, M. Shi, Y. Du, W. Wang and Y. Zhang, Mechanisms of Cdc42-mediated rat MSC differentiation on micro/nano-textured topography, Acta Biomater., 2017, 49, 235–246 CrossRef CAS PubMed.
- I. Ullah, R. B. Subbarao and G. J. Rho, Human mesenchymal stem cells – current trends and future prospective, Biosci. Rep., 2015, 35(2), pii: e00191, DOI:10.1042/BSR20150025.
- S. H. Carroll and K. Ravid, Differentiation of mesenchymal stem cells to osteoblasts and chondrocytes: a focus on adenosine receptors, Expert Rev. Mol. Med., 2013, 15, e1 CrossRef PubMed.
- P. R. Angelova, A. Y. Baev, A. V. Berezhnov and A. Y. Abramov, Role of inorganic polyphosphate in mammalian cells: from signal transduction and mitochondrial metabolism to cell death, Biochem. Soc. Trans., 2016, 44, 40–45 CrossRef CAS PubMed.
- M. Neufurth, X. H. Wang, H. C. Schröder, Q. L. Feng, B. Diehl-Seifert, T. Ziebart, R. Steffen, S. F. Wang and W. E. G. Müller, Engineering a morphogenetically active hydrogel for bioprinting of bioartificial tissue derived from human osteoblast-like SaOS-2 cells, Biomaterials, 2014, 35, 8810–8819 CrossRef CAS PubMed.
- X. Wang, S. Xu, S. Zhou, W. Xu, M. Leary, P. Choong, M. Qian, M. Brandt and Y. M. Xie, Topological design and additive manufacturing of porous metals for bone scaffolds and orthopaedic implants: a review, Biomaterials, 2016, 83, 127–141 CrossRef CAS PubMed.
- J. Y. Rho, L. Kuhn-Spearing and P. Zioupos, Mechanical properties and the hierarchical structure of bone, Med. Eng. Phys., 1998, 20, 92–102 CrossRef CAS PubMed.
- P. Camper, Naauwkeurige afbelding en beschryving van eene geheel en al verloorene, maar door kunst herstelde neus en verhemelte, Seep-Boekverkooper, Amsterdam, 1771 Search PubMed.
- N. Senn, Senn on the healing of aseptic bone cavities by implantation of antiseptic decalcified bone, Ann. Surg., 1889, 10, 352–368 CrossRef.
- M. J. Yaszemski, R. G. Payne, W. C. Hayes, R. Langer and A. G. Mikos, Evolution of bone transplantation: molecular, cellular and tissue strategies to engineer human bone, Biomaterials, 1996, 17, 175–185 CrossRef CAS PubMed.
- C. X. Lam, D. W. Hutmacher, J. T. Schantz, M. A. Woodruff and S. H. Teoh, Evaluation of polycaprolactone scaffold degradation for 6 months in vitro and in vivo, J. Biomed. Mater. Res., Part A, 2009, 90, 906–919 CrossRef PubMed.
- D. T. Butcher, T. Alliston and V. M. Weaver, A tense situation: forcing tumour progression, Nat. Rev. Cancer, 2009, 9, 108–122 CrossRef CAS PubMed.
- P. Lu, V. M. Weaver and Z. Werb, The extracellular matrix: a dynamic niche in cancer progression, J. Cell Biol., 2012, 196, 395–406 CrossRef CAS PubMed.
- D. G. Hardie and M. L. Ashford, AMPK: regulating energy balance at the cellular and whole body levels, Physiology, 2014, 29, 99–107 CrossRef CAS PubMed.
- D. G. Hardie, AMP-activated protein kinase: an energy sensor that regulates all aspects of cell function, Genes Dev., 2011, 25, 1895–1908 CrossRef CAS PubMed.
- E. Dornier and J. C. Norman, Tensin links energy metabolism to extracellular matrix assembly, J. Cell Biol., 2017, 216, 867–869 CrossRef CAS PubMed.
- L. D. Muiznieks and F. W. Keeley, Molecular assembly and mechanical properties of the extracellular matrix: A fibrous protein perspective, Biochim. Biophys. Acta, 2013, 1832, 866–875 CrossRef CAS PubMed.
- G. C. Yeo, F. W. Keeley and A. S. Weiss, Coacervation of tropoelastin, Adv. Colloid Interface Sci., 2011, 167, 94–103 CrossRef CAS PubMed.
- A. E. Vercesi, S. N. Moreno and R. Docampo, Ca2+/H+ exchange in acidic vacuoles of Trypanosoma brucei, Biochem. J., 1994, 304, 227–233 CrossRef CAS PubMed.
- W. E. G. Müller, E. Tolba, H. C. Schröder, B. Diehl-Seifert and X. H. Wang, Retinol encapsulated into amorphous Ca2+ polyphosphate nanospheres acts synergistically in MC3T3-E1 cells, Eur. J. Pharm. Biopharm., 2015, 93, 214–223 CrossRef PubMed.
- W. E. G. Müller, M. Neufurth, E. Tolba, S. Wang, W. Geurtsen, Q. Feng, H. C. Schröder and X. H. Wang, A biomimetic approach to ameliorate dental hypersensitivity by amorphous polyphosphate microparticles, Dent. Mater., 2016, 32, 775–783 CrossRef PubMed.
- M. Neufurth, X. H. Wang, E. Tolba, B. Dorweiler, H. C. Schröder, T. Link, B. Diehl-Seifert and W. E. G. Müller, Modular small diameter vascular grafts with bioactive functionalities, PLoS One, 2015, 10(7), e0133632, DOI:10.1371/journal.pone.0133632.
- W. E. G. Müller, S. Wang, M. Wiens, M. Neufurth, M. Ackermann, D. Relkovic, M. Kokkinopoulou, Q. Feng, H. C. Schröder and X. H. Wang, Uptake of polyphosphate microparticles in vitro (SaOS-2 and HUVEC cells) followed by an increase of the intracellular ATP pool size, PLoS One, 2017, 12(12), e0188977, DOI:10.1371/journal.pone.0188977.
- H. C. Schröder, X. H. Wang and W. E. G. Müller, Polyphosphat-Nano/Mikropartikel – Energiereiche anorganische Polymer-Partikel für innovative Anwendungen in der Medizin, GIT-Labor [Portal für Anwender in Wissenschaft und Industrie], 2017, 61, 30–32 Search PubMed.
- K.-W. Lee, N. R. Johnson, J. Gao and Y. Wang, Human progenitor cell recruitment via SDF-1α coacervate-laden PGS vascular grafts, Biomaterials, 2013, 34, 9877–9885 CrossRef CAS PubMed.
- D. F. Williams, The essential materials paradigms for regenerative medicine, J. Mater., 2011, 63, 51–55 Search PubMed.
- D. F. Williams, A paradigm for the evaluation of tissue-engineering biomaterials and templates, Tissue Eng., Part C, 2017, 23, 926–937 CrossRef PubMed.
- D. Tang, R. S. Tare, L. Y. Yang, D. F. Williams, K. L. Ou and R. O. Oreffo, Biofabrication of bone tissue: approaches, challenges and translation for bone regeneration, Biomaterials, 2016, 83, 363–382 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2018 |