Core–shell protein clusters comprising haemoglobin and recombinant feline serum albumin as an artificial O2 carrier for cats†
Abstract
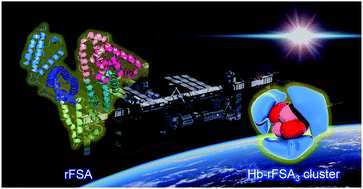
This report describes the synthesis and structure of core–shell protein clusters comprising haemoglobin (Hb) at the centre and recombinant feline serum albumin (rFSA) at the exterior, named as haemoglobin–albumin clusters (Hb–rFSA3). Specifically, we highlight their capability as an artificial O2 carrier that can be used as a red blood cell (RBC) substitute for cats, the most populous pet animal in the world. First, rFSA was expressed by genetic engineering using Pichia yeast. The proteins show identical features to the native FSA derived from feline plasma. Single crystals of rFSA were prepared under a microgravity environment on the international space station (ISS), from which the structure was first revealed at 3.4 Å resolution. Subsequently, bovine Hb was wrapped covalently by rFSA using an α-succinimidyl-ε-maleimide crosslinker, yielding Hb–rFSA3 clusters. Three rFSA entities enfolded the Hb nuclei satisfactorily, giving the protein clusters a negative surface net charge (pI = 4.7) and preventing an immunological response against anti-Hb antibodies. The O2 affinity was higher (P50 = 9 Torr) than that of the native Hb. The Hb–rFSA3 clusters are anticipated for use as an alternative material for RBC transfusion, and as an O2 therapeutic reagent that can be exploited in various veterinary medicine scenarios.


 Please wait while we load your content...
Please wait while we load your content...