Revealing the chemistry of an anode-passivating electrolyte salt for high rate and stable sodium metal batteries†
Abstract
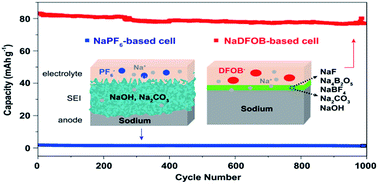
Stabilizing the reactive metal anode is a critical challenge for the development of next-generation alkali metal batteries. This work demonstrates that sodium-difluoro(oxalato)borate (NaDFOB)-based carbonate-ester electrolytes have excellent compatibility with the anode in sodium metal batteries, enabling high rate performance and long cycle life. The NaDFOB-based electrolytes possess favourable electrochemical stability and effectively passivate the Na metal anode by forming a compact, robust and conductive solid-electrolyte interphase (SEI) layer. Quantitative nuclear magnetic resonance (NMR) measurements of electrolyte solvents indicated that the SEI layer forms mainly in the initial cycles, and it prevents further solvent degradation. Solid-state NMR and X-ray photoelectron spectroscopy studies revealed the chemical composition of the NaDFOB-derived SEI film, which includes a mixed phase consisting primarily of sodium diborate, tetrafluoroborate and carbonate. The formation of such a composite SEI rich in borate and tetrafluoroborate provides robust structural and chemical stability, and facilitates fast ion transport for uniform Na stripping/plating.


 Please wait while we load your content...
Please wait while we load your content...