Insight into fast Li diffusion in Li-excess spinel lithium manganese oxide†
Abstract
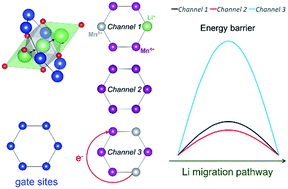
Li-excess cathode materials are expected to have great potential for applications in lithium-ion batteries owing to their high energy density. In addition to the extensive studies on the anionic redox activity in lithium-ion batteries, their Li-ion diffusion properties have also attracted much interest. Using ab initio calculations, herein, we systematically explored Li diffusion properties in both stoichiometric and Li-excess phases of spinel lithium manganese oxide (LMO). Our results showed that there is a type of structural unit (six Mn ions forming a cation ring for Li-ions to pass through during migration) that acts as “gate sites” and the Li-excess configuration could introduce two types of fast Li-ion migration channels to enhance the Li-ion diffusivity. The first type of fast channels resulted from the decreased repulsive coulombic interactions between the cations at the gate site and the mobile Li-ions due to the substitution of Mn3+ by Li+. The second type of fast channels originated because the excess Li could induce more gate sites with symmetrical distribution of Mn4+ surrounding the Li diffusion channel, which is proved to be able to enhance the Li-ion mobility. Interestingly, it was also found that in slow Li diffusion channels for both stoichiometric and Li-excess LMO, a simultaneous polaron hopping process around the gate sites would be coupled with the Li migration process, which accounts for the high energy barriers for Li-ion diffusion.


 Please wait while we load your content...
Please wait while we load your content...