A theoretical study on the surface and interfacial properties of Ni3P for the hydrogen evolution reaction†
Abstract
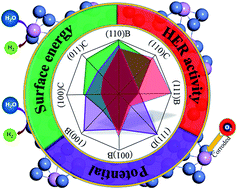
We report a comprehensive density functional theory (DFT) study on the stability, geometric structure, electronic characteristics, and catalytic activity for the hydrogen evolution reaction (HER) on low-index Ni3P crystal surfaces, namely, the (001), (100), (110), (101) and (111) planes with different surface terminations. The results indicate that P-rich and some stoichiometric surfaces are thermodynamically stable. Eight stable surfaces were selected to investigate the electronic characteristics and catalytic activity. The (110)B facet of Ni3P is indispensable for the HER, because it not only displays improved electrocatalytic activity, but also possesses suitable potential and high stability. Increasing the active sites through doping or enlarging the surface area could be a useful strategy to improve the HER activity further. Furthermore, it was found that Ni3P requires higher energies for decomposition in the absence of O2, although it is thermodynamically unstable in aqueous solutions with most pH values and potentials. This study provides important insights into the surface properties of Ni3P for water splitting and opens up an exciting opportunity to optimize the performance of solar energy conversion devices by synthesizing preferentially exposed catalyst facets.
- This article is part of the themed collection: 2018 Journal of Materials Chemistry A HOT Papers


 Please wait while we load your content...
Please wait while we load your content...