Ultra-high-rate, ultra-long-life asymmetric supercapacitors based on few-crystalline, porous NiCo2O4 nanosheet composites†
Abstract
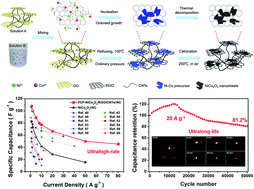
NiCo2O4-based supercapacitors are promising for practical applications due to their high specific capacitance, good electrical conductivity and electrochemical activity. Significant advances are highly desired but few studies can simultaneously achieve good rate capability and long cycle life (>30 000 cycles) while maintaining high specific capacitance, three factors that dominate practical applications. Here, we demonstrate that when few-crystalline, porous NiCo2O4 nanosheets grown on graphene and carbon nanotubes were assembled into asymmetric supercapacitors, they delivered an energy density of 38.1 W h kg−1 at 797.8 W kg−1 or 13.3 W h kg−1 at 58.1 kW kg−1. Even at a current density of 20 A g−1, they retained 104.5% and 81.2% of the initial capacitance after 20 000 and 50 000 cycles, respectively. Such performance arises from a new strategy that balances the surface active site, ion diffusion, charge transfer and structural stability of active materials during high-rate cycling. Different from well-crystalline or amorphous pseudocapacitive materials, the few-crystalline, porous nanosheets not only afford an effective charge transfer path when combining with conductive graphene and carbon nanotubes, but also prevent the aggregation of conductive components and boost the diffusion of electrolyte ions within the electrode. The presence of the amorphous domains enriches the surface active sites of nanosheets and enhances their adaptability to deformation during large-current charge/discharge. This strategy holds promise for addressing critical issues facing pseudocapacitive materials, enabling the optimization of the comprehensive performance of supercapacitors in a mild manner.


 Please wait while we load your content...
Please wait while we load your content...