Modifying the electrochemical performance of vertically-oriented few-layered graphene through rotary plasma processing†
Abstract
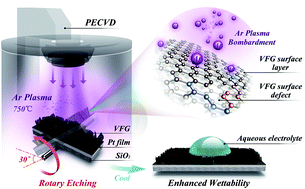
Vertically-oriented few-layered graphene (VFG) has a unique three-dimensional morphology and exposed ultrathin edges, and shows great promise for high-performance supercapacitor applications. However, VFG shows limited capacitance owing to poor wettability with electrolytes, which has been a bottleneck for further applications. Herein, we designed and developed an effective strategy, based on rotary plasma etching, to create defects on the side surfaces while simultaneously maintaining the structural integrity of VFG. Rotary plasma etching decreased the contact angle (CA) of VFG from 123° to 34°, compared with conventional vertical etching, which only reduced the CA to 71°. Electrochemical studies demonstrated that VFG samples with a high density of surface defects, introduced by rotary plasma etching, exhibited a high areal capacitance of 1367 μF cm−2 (a volumetric specific capacitance of 137 F cm−3), which was approximately 4 times as large as for pristine VFG-based materials. Our study offers feasible insight into the use of an industrially viable method, rotary plasma processing, for modifying and enhancing the properties of VFG. Our findings may help to accelerate the development of more effective energy storage devices.


 Please wait while we load your content...
Please wait while we load your content...