Competitive calcium ion binding to end-tethered weak polyelectrolytes†
Abstract
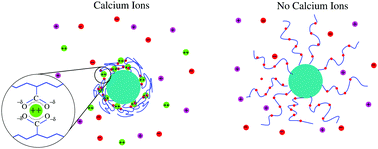
We have developed a molecular model to describe the structural changes and potential collapse of weak polyelectrolyte layers end-tethered to planar surfaces and spherical nanoparticles as a function of pH and divalent ion concentration. In particular, we describe the structural changes of polymer-coated nanoparticles end-tethered to copolymers of poly(acrylic acid) (pAA) and poly arcrylamido-2-methylpropane sulfonate (pAMPS) in the presence of Ca2+ ions. We find that end-grafted poly(acrylic acid) layers will collapse in aqueous solutions containing sufficient amounts of Ca2+ ions, while polymers and copolymers with sufficient AMPS monomers will not collapse. The collapse of end-tethered pAA is due to the formation of calcium bridges between two acrylic acid monomers and one calcium ion. On the other hand pAMPS layers do not collapse due to the lack of calcium bridges. The collapse of pAA layers is strongly dependent on the pH as well as divalent and monovalent salt concentrations of the environment. The collapse is also strongly influenced by the curvature of the tethering surface.


 Please wait while we load your content...
Please wait while we load your content...