Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Closing the carbon cycle to maximise climate change mitigation: power-to-methanol vs. power-to-direct air capture
H. A.
Daggash
 abc,
C. F.
Patzschke
d,
C. F.
Heuberger
abc,
C. F.
Patzschke
d,
C. F.
Heuberger
 bc,
L.
Zhu
c,
K.
Hellgardt
c,
P. S.
Fennell†
d,
A. N.
Bhave
e,
A.
Bardow
bc,
L.
Zhu
c,
K.
Hellgardt
c,
P. S.
Fennell†
d,
A. N.
Bhave
e,
A.
Bardow
 f and
N.
Mac Dowell‡
f and
N.
Mac Dowell‡
 *bc
*bc
aGrantham Institute for Climate Change and the Environment, Imperial College London, Exhibition Road, London, SW7 2AZ, UK
bCentre for Environmental Policy, Imperial College London, Exhibition Road, London, SW7 2AZ, UK. E-mail: niall@imperial.ac.uk; Tel: +44 (0)20 7594 9298
cCentre for Process Systems Engineering, Imperial College London, Exhibition Road, London, SW7 2AZ, UK
dDepartment of Chemical Engineering, Imperial College London, Exhibition Road, London, SW7 2AZ, UK
eCMCL, Salisbury House, Station Road, Cambridge, CB1 2LA, UK
fLehrstuhl für Technische Thermodynamik, RWTH Aachen University, 52062 Aachen, Germany
First published on 27th April 2018
Abstract
It is broadly recognised that CO2 capture and storage (CCS) and associated negative emissions technologies (NETs) are vital to meeting the Paris agreement target. The hitherto failure to deploy CCS on the required scale has led to the search for options to improve its economic return. CO2 capture and utilisation (CCU) has been proposed as an opportunity to generate value from waste CO2 emissions and improve the economic viability of CCS, with the suggestion of using curtailed renewable energy as a core component of this strategy. This study sets out to quantify (a) the amount of curtailed renewable energy that is likely to be available in the coming decades, (b) the amount of fossil CO2 emissions which can be avoided by using this curtailed energy to convert CO2 to methanol for use as a transport fuel – power-to-fuel, with the counterfactual of using that curtailed energy to directly remove CO2 from the atmosphere via direct air capture (DAC) and subsequent underground storage, power-to-DAC. In 2015, the UK curtailed 1277 GWh of renewable power, or 1.5% of total renewable power generated. Our analysis shows that the level of curtailed energy is unlikely to increase beyond 2.5% until renewable power accounts for more than 50% of total installed capacity. This is unlikely to be the case in the UK before 2035. It was found that: (1) power-to-DAC could achieve 0.23–0.67 tCO2 avoided MWh−1 of curtailed power, and (2) power-to-Fuel could achieve 0.13 tCO2 avoided MWh−1. The power-to-fuel concept was estimated to cost $209 tCO2 avoided−1 in addition to requiring an additional $430–660 tCO2 avoided−1 to finally close the carbon cycle by air capture. The power-to-DAC concept was found to cost only the $430–660 tCO2 avoided−1 for air capture. For power-to-fuel to become profitable, hydrogen prices would need to be less than or equal to $1635 tH2−1 or methanol prices must increase to $960 tMeOH−1. Absent this change in H2 price or methanol value, a subsidy of approximately $283 tCO2−1 would be required. A core conclusion of this study is that using (surplus) renewable energy for direct air capture and CO2 storage is a less costly and more effective option to mitigate climate change than using this energy to produce methanol to substitute gasoline.
1 Introduction
Whilst the 2015 Paris agreement symbolised the recognition of climate change as a global issue and achieved political consensus to limit its consequences, mainstream climate policy is yet to evolve to promote the proposed mitigation strategies required to achieve the agreed well below 2 °C warming (above pre-industrial levels) target. Integrated assessment models (IAMs) are reliant on the deployment of CCS and negative emissions technologies (NETs) to meet this target.1–6 Despite the prevalence of CCS in all mitigation pathways compliant with the Paris target, high investment and operating costs, and cross-chain risks have deterred its deployment at the required scale.7,8 An absence of financial incentives, policy drivers and/or political appetite has compounded the investment challenges of developing CCS infrastructure. These challenges have driven the search for means to improve the economic viability of CCS, one of which is carbon dioxide (CO2) capture and utilisation (CCU).9,10The appeal of CCU lies in its alleged potential to valorise CO2 by converting waste emissions into valuable products. CCU comprises both capture processes from industrial and diffuse CO2 sources, and their direct or indirect utilisation. Approximately 0.5% of global CO2 emissions is currently utilised, mostly for enhanced oil recovery (EOR) or the synthesis of urea and speciality chemicals.11,12 Some routes for CO2 utilisation are currently commercialised, such as CO2-based polymers.13 It is further argued that CCU could serve to make CCS more financially attractive by offsetting investment costs with revenue from CO2-derived products.13
Decarbonising the transport sector, which accounts for approximately 14% of total anthropogenic GHG emissions, is considered difficult because of the multitude of small, mobile emitters.1 Additionally, with global fuel consumption two orders of magnitude higher than that of chemicals, CCU for the production of transport fuels has been promoted as providing an attractive opportunity for simultaneous value creation and emissions reduction.7,14 Moreover, owing to the increasing deployment of intermittent renewable energy sources, it is argued that renewable energy that would otherwise be curtailed can be used to operate these CCU processes, with this energy, available at zero or even negative cost.15
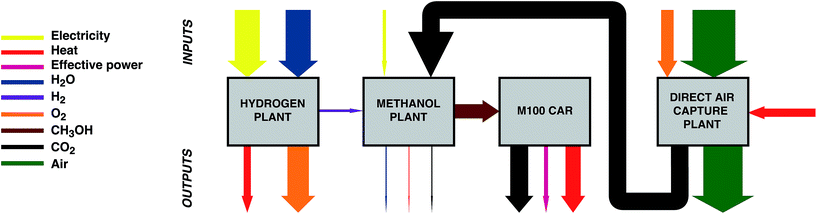
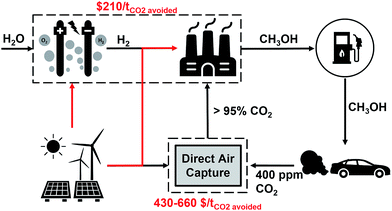
This study sets out to quantify: (a) the amount of curtailed renewable energy that is likely to be available in the medium term, (b) the extent to which transport-related CO2 emissions can be avoided via a power-to-fuel strategy, and (c) to contrast this with the counterfactual argument of using the otherwise curtailed renewable energy to directly remove CO2 from the atmosphere via a direct air capture (DAC) process. The power-to-fuel scenario utilises curtailed renewable electricity to produce H2 which is subsequently reacted with CO2 to produce methanol as a gasoline substitute. The CO2 emissions from the methanol-powered cars are recycled for the continued production of methanol via direct air capture (DAC). In contrast, power-to-DAC uses the electricity to operate a DAC plant which directly removes CO2 from the atmosphere; the captured CO2 is then permanently sequestered. Both process are illustrated in Fig. 1.
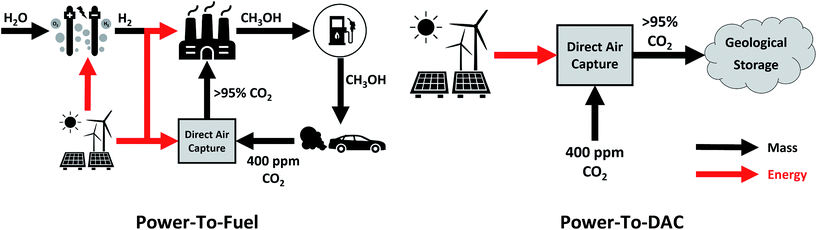 | ||
| Fig. 1 Conceptual diagrams of the proposed circular economy of power-to-Fuel (left) and power-to-DAC (right) processes. | ||
2 Key enabling factors
2.1 Curtailed renewable energy
While CCS deployment has, as yet, failed to materialise at scale, mitigation efforts have, however, resulted in an increasing penetration of renewables into the electricity system. The relative unpredictability of their supply poses risks to grid stability and reliability, and their increased deployment may see a manifestation of those risks.16 The various technical requirements for grid stability and security of supply are discussed elsewhere,17 and are not repeated here. Surplus generation during low-demand hours and full storage capacity, coupled with the difficulty of rapidly ramping (up or down) firm low-carbon electricity generation will likely see an increase in constraint payments already made to wind farms to curtail their generation. The opportunity for developing CO2 utilisation processes using surplus electricity from intermittent renewables energy sources (iRES) depends on the quantity of this surplus, which is spatially and temporally varying. It is therefore helpful to review recent experience with curtailment of power generation from iRES in various power systems and curtailment analyses with energy system models.Public and research literature do not provide a unique definition for curtailment in power systems. Curtailment levels are reported as the amount of downwards regulated power from iRES§ per maximum potential power generation from iRES,18 per total system-wide power generation, or per total electricity demand.19 These different definitions lead to different numerical value, hence curtailment levels should be compared with caution.
Curtailment of power generation from iRES is a security measure required to maintain power system stability and operability, and to prevent equipment damage.20 The reasons for curtailment depend on the specific conditions of a given network with regards to generation, energy storage, and transmission capacity.21 Often a surplus of power generation from iRES or conventional units during periods of low demand is the cause, but prevention or mitigation of transmission congestion (e.g., in the United States (US) MISO network) and/or initial lack of transmission capacity (e.g., China, US ERCOT) may make curtailment necessary.18,22 As observed in the Irish power system, exogenous operating requirements, e.g., a minimum share of firm reserve capacity,¶ can also result in curtailment of power generation from iRES.18
iRES are near-zero marginal cost units, consequently, reduced generation (because of curtailment) is accompanied by a cost increase for electricity (per MWh).22 However, certain levels of curtailment can be economically sensible, reducing start-up cost and cycling for conventional power plants, or costly balancing mechanisms for transmission system operation. A dynamic system-wide economic dispatch assessment is necessary to evaluate the most economical and technically feasible operational strategy.23
The analysis of historic power system data reveals curtailment levels of below 10% up to iRES penetration levels of 50% (as a percentage of available iRES power generation) in the UK, Ireland, and other European countries. A few sources report levels up to 20% at penetration levels exceeding 60%.22 In some US and Chinese power networks higher curtailment levels are observed, increasing above 20% at iRES penetration levels below 10%. Bird et al., however, show that such historically high levels in the US were due to transmission bottlenecks and have since fallen to a range between 2–4%.18 The review18 presents a study of wind curtailment levels and causes across Europe, China, and the US for 2013 reports an overall average range of 1–3%. Where renewable energy is curtailed, those generators who are being constrained off the grid are compensated for this. For example, during Easter 2016, a total of $5.3 million was paid to 39 UK wind farms, with constraint prices at $83–225 MWh−1.||**25 Therefore, it is unlikely that this energy will be available at zero or negative cost to those wishing to use it.
Reduced incidences of curtailment can be achieved through increased power system flexibility, e.g., through increased energy storage capacity or an expansion/reinforcement of the existing transmission grid. Changes in the electricity market and regulatory frameworks, as well as improved forecasting and dispatch strategies can equally alleviate high curtailment levels.18 The participation of iRES power generation units in ancillary services provides additional integration potential. The provision of upward reserve service requires large amounts of iRES power to be curtailed pre-emptively, which is not attractive under current market schemes.26 However, the provision of downward reserve as well as voltage control in the case of wind power plants is technically possible and meaningful where market incentives are given. Additionally, if wind farms operate in a coordinated way, they may have the potential to provide secondary frequency control and so further reduce curtailment levels.26
Besides the analysis of real-word data, the energy systems modelling community has been studying curtailment in current and future power systems. A detailed study of the European electricity system including energy storage capacity expansion as well as transmission grid re-enforcement with the ReMix model reveals that even at penetration levels of 40–50% of iRES capacity not more than 1% of annual power demand is curtailed.19 As the iRES share increases to 60%, curtailment levels remain below 25% of annual power demand. It is observed that curtailment levels increase more rapidly in solar-dominated power systems. The PLEXOS model has been used to explore wind curtailment in a potential 2020 Irish power system.27 The study reports transmission constraints as the primary driver of curtailment and finds a reduction in curtailment levels from 14 to 7% as reserve requirements (SNSP) are adjusted. Several measures are being pursued to minimise curtailment in regional power systems: automatic generation control28 and expansion of the energy imbalance market,29 amongst others, in California; grid expansion and award of priority to clean power feed in China;30 flexibilisation of conventional power plants and expansion of regional transmission networks in Denmark;31etc. These will serve to keep curtailment at or below contemporary levels. Thus, whilst it is evident that instances of surplus renewable energy have existed in different regions, going forward, curtailment is likely to be reduced.
We now present an iRES power curtailment analysis with the Electricity Systems Optimisation (ESO) modelling framework.23 The ESO model is a hybrid capacity expansion and unit commitment model based on a total cost minimising mixed-integer linear program. The ESO model determines the optimal dispatch strategy on a hourly scale for one year without foreknowledge. The high temporal resolution combined with the detailed unit-wise representation of thermal, iRES, and energy storage technologies makes the ESO model well suited for analysis. As the national transmission grid is not considered in this model, the derived curtailment levels are due to demand-supply imbalances, technical operational constraints, and ancillary service constraints. The ESO model is parametrised to the power systems of the United Kingdom (UK) based on projections for 2030.32
Similarly to previous studies, the analysis with the ESO model confirms low levels of curtailment of power generation from iRES as percentage of available iRES power generation at iRES penetration levels below 40%. Fig. 2 illustrates iRES power curtailment as a function of their penetration as output from the ESO model. We find that only at penetration levels above 50–60% do curtailment levels of greater than 2.5% occur. In a UK context, these values convert to an annual total of 11–77 TWh, approximately 3–19% of electricity demand in 2030. Based on BEIS′ reference scenario and the CCC's central scenario of the UK's electricity generation mix, it is unlikely that the share of intermittent renewables will reach such levels by 2035.38,39
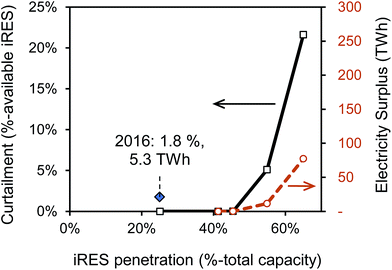 | ||
| Fig. 2 Curtailment of intermittent renewable energy sources (iRES), i.e., onshore wind, offshore wind, solar, as a function of the iRES capacity share in a potential 2030 power system of the United Kingdom. Curtailment (blue, left y-axis) and energy surplus (green, right y-axis) data is obtained with the Electricity System Optimisation (ESO) model,23,33 which determines optimal electricity dispatch strategy hourly for one year, and does not take transmission constraints into account. Availability data for iRES are taken from ref. 34–36. Curtailment data point for 2016 is derived from National Grid's monthly balancing service reports throughout 2016 referring to total constrained volume including non-iRES fuel types.37 | ||
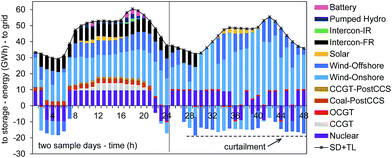
Fig. 3 explores the optimal dispatch strategy for two sample days in the 2030 UK power system as determined with the ESO model. We can follow the individual power unit operation, storage charging and discharging, shown here aggregated by fuel type. In the 24 hour sequence on the left hand-side, no iRES generated power is curtailed; in the night time, however, energy storage is charged with excess power from onshore and offshore wind power. In the sample day on the right hand-side, power availability from iRES is high and again energy storage is utilised. However, as the maximum storage capacity is reached a curtailment of iRES power is economically sensible, avoiding the turn-down/shut-down of nuclear power stations. Overall, we observe higher curtailment levels at night times than during the day. Flexibility of operation is therefore crucial for a process seeking to utilise this surplus electricity. Consequently, several technical/engineering challenges arise. These are discussed in detail in Section 5.
The ESO modelling framework provides the cost-optimal dispatch pattern for electricity generation, but does not account for lack of grid operator expertise or grid congestion. There is evidence for the UK that constraint payments are still being made to wind farm operators to turn-down their generation, implying that accounting grid congestion and operator expertise are important in reliably predicting the extent to which renewable energy might be curtailed in the future. Curtailed power, therefore, will remain a limited resource for the foreseeable future which should be used in the most efficient way to maximise climate change mitigation.40 The present studies focuses on power-to-methanol and power-to-DAC as two options for direct and indirect emission-reductions of the transportation sector. In practice, these routes will further have to compete with grid expansion and other options to utilize electricity (e.g., power-to-heat).40
2.2 Non-fossil CO2
Without final capture and reuse of the CO2 arising from combustion, the production of CO2-derived methanol requires the continued extraction of fossil fuels, thus locking us into partial decarbonisation scenarios, quite at odds with the Paris agreement. It is therefore vital that the carbon cycle is closed, and that these emissions are captured and reused. The direct air capture of CO2 is potentially well-suited for this task. Furthermore, its prospective ease of scale-up (in the case of increased curtailment) owing to its potentially modular nature41–43 provides an added advantage.Direct removal of CO2 from the air bears similarities to post-combustion capture processes employed by CCS technologies as both are based on chemical absorption. The lower concentrations of CO2 in air compared to power plant exhaust gases (0.04% and 3–12%, respectively) result in an increased energy cost of capture and render amine solvents unsuitable as they react with the CO2 in air too slowly.
The majority of academic literature on DAC focuses on hydroxide-based capture, often using sodium or potassium hydroxide as a capture solution. Energetic analyses of proposed DAC technologies specify a wide range of energy costs for capture ranging from 6.7 to 22.7 GJ tCO2−1, with the upper bound estimates bringing the technical and economic feasibility of the process into question.42,44–47
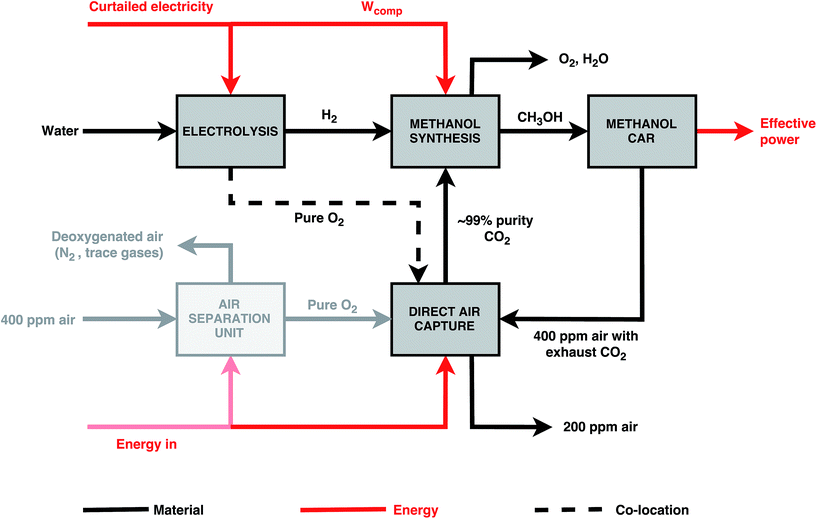
In this study, the DAC plant assumes a potassium hydroxide (KOH) process, as previously described by Keith48et al. and Baciocchi.45 It is illustrated in Fig. 4. KOH solution is contacted with ambient air in an absorber where it reacts with the CO2 in the air to form a carbonate, K2CO3. Calcium carbonate fed into a kiln is then calcined at 900–1000 °C to produce calcium oxide (CaO) which is subsequently hydrated to obtain calcium hydroxide (Ca(OH)2). CO2 is produced as a result and is separated for sequestration or CCU purposes. The Ca(OH)2 produced is reacted with K2CO3 to regenerate the capture solution and the cycle is repeated. A high-grade heat input to the kiln is required by the process shown in Fig. 4. The produced CO2 stream is available at approximately 900 °C – heat is therefore recovered and used to generate electricity which meets the outstanding energy needs of the plant.42 This process can be operated using either natural gas or electricity only.42 As only curtailed electricity is assumed to be available in this study, an electricity-driven DAC plant is assumed.††
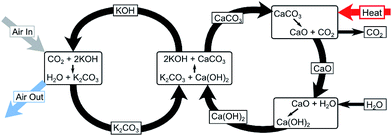 | ||
| Fig. 4 Chemical reactions involved in the capture of CO2 directly from the atmosphere using a potassium hydroxide (KOH) solution.42 | ||
DAC technology is still in the early stages of research and development, and thus evaluating a potential range of energy costs is vital. The lower and upper bound values used in this work were taken from the Carbon Engineering DAC pilot project42 and a process design proposed by the American Physical Society, respectively. These processes were selected owing to availability of adequate information for this analysis. Both designs employ hydroxide-based capture as described by Fig. 4. Their heat, electricity and equivalent primary energy requirements are provided in Table 1.
2.3 Green hydrogen
Conventionally, hydrogen is obtained by steam reforming of methane/natural gas. However, to minimise the lifecycle emissions associated with the power-to-fuel process, hydrogen must be obtained from a renewable source. The production of electrolytic hydrogen uses a commercially available technology that can be powered with renewable energy. The theoretical energy demand of the electrolyser is equal to the reaction enthalpy, ΔH. It comprises the changes in Gibbs free energy and entropy, ΔG and ΔT.S, which represent the electricity and heat requirements, respectively, hence increases with temperature. In reality a considerable amount of electrical energy is usually converted into heat energy, due to joule heating of the electrical current which flows through the cell.49 ΔG therefore decreases with increasing operating temperature.50 In this study, curtailed renewable electricity is the selected energy source, hence lower temperature processes are economically favourable. Consequently, low temperature electrolysers (LTEs) have been used in this study. The most common LTEs are alkaline water (AE) and proton-exchange membrane (PEM) electrolysers. Their operating parameters are provided in Table 2.The relative simplicity of AEs results in the relatively low capital and operating cost compared to PEMs.49 The limited H2 production rate by PEMs makes AEs doubly advantageous hence they have been used in this study. In alkaline water electrolysis, two electrodes are submerged in a NaOH or KOH solution separated by a membrane, which is only permeable to water and hydroxide. Hydrogen forms at the cathode when electrons are supplied by an external power source. The remaining hydroxide ions migrate through the cell membrane to the anode. A disadvantage of this set-up is the considerable ohmic losses that occur when the electrical current passes through the cell. Furthermore, the membrane limits the maximum current density. Since the separation efficiency of the membrane is not perfect, minor amounts of oxygen will diffuse back into the cathode chamber where it reacts with H2 to reform water, thereby reducing the process efficiency. Hydrogen diffusion back into the anode chamber occurs especially at lower loads (<40%).52 This results in lower system dynamics, which may be a major drawback given the fluctuating nature of the wind energy supplied. However, modern AEs such as the A-range series from NEL hydrogen are optimised to run with wind energy and hence allow flexible, efficient and safe operation across the operating range of 15–100% of the maximum capacity.53
Taking into account the energy dissipated due to system irreversibilities, the molar energy consumption for hydrogen production is 378.9 kJ mol−1 (4.7 kW h N−1 m−3 or 189.5 MJ kg−1).54 Recent breakthroughs have however improved the efficiency of alkaline water electrolysers. The NEL A-485 manufactured by NEL hydrogen can produce 99.9% (±0.1%) purity H2 at 485 N m3 h−1 with a DC power consumption of just 3.8 kW h N−1 m−3.53
3 Power-to-fuel
3.1 Methanol as a fuel
The methanol economy, as proposed by Asinger55 and later by Olah,56,57 presents methanol as a ready substitute for transport fuels, suitable energy storage medium and raw material for the production of synthetic hydrocarbons. In internal combustion engines (ICEs), methanol can be used either as a fuel-blend or as a dedicated (100%) fuel, in both spark ignition (SI) and compression ignition (CI) operating modes. While its relative ease of handling at ambient conditions and suitability for ICEs with little modifications are advantageous, clarity on the amount of methanol required to displace fuel for a given purpose is crucial to understanding its potential.Table 3 highlights the properties of both fuels. While methanol is denser and emits 1.7 kgCO2 kgfuel−1 less than gasoline, it is inferior if energy density is considered. To determine the amount of methanol needed to displace gasoline, the energy normalisation factor (ENF) and volume normalisation factor (VNF), defined below, are employed:
 | (1) |
 | (2) |
| Fuel | Mass density (kg L−1) | Lower heating value (MJ kgfuel−1) | Carbon density (kgCO2 kgfuel−1) | Carbon density (kgCO2 MJ−1) |
|---|---|---|---|---|
| a Gasoline typically contains a mixture of C4–C12 hydrocarbons. The combustion characteristics of octane (C8H18) have been used to estimate the carbon density of gasoline. | ||||
| Methanol | 0.79 | 19.7 (ref. 11) | 1.375 | 0.070 |
| Gasoline | 0.75 | 46.4 (ref. 11) | 3.088a | 0.067 |
The equivalence of methanol and gasoline is given by a product of the above, i.e. for each litre of gasoline consumed, 2.23 litres of methanol are required to provide the same energy service. Drivers of methanol cars will therefore have to refuel more than twice as often compared to those using gasoline-powered cars. This puts the carbon intensity of using these fuels for transport in perspective—while methanol has significantly lower CO2 emissions (per kg of fuel) compared to gasoline, its relatively low energy density means that 3.25 gCO2 more are emitted per MJ of energy service provided; a 5% increase relative to the gasoline baseline.
However, there is more to engine performance than simply the energy content of the fuel. Methanol has a relatively high octane number (RON = 108.7), high latent heat of vaporisation (1103 kJ kg−1) and higher oxygen content (50% by mass) compared to gasoline. Blending it with gasoline therefore increases the octane number of the fuel-blend, allowing for higher compression ratios and increased thermal efficiency of the system. A study investigating the effect of pure methanol over a gasoline engine at different compression ratios reported higher torque output, cylinder output, power output and specific fuel consumption at higher compression ratios.58 The high heat of vaporisation leads to a reduction of the temperature of the incoming fuel–air charge, thus increasing the volumetric efficiency and in turn, the power output. The reduced combustion temperatures can lead to simultaneous low soot and NOx emissions.59,60 Reduced soot and smoke emissions also result due to the higher oxygen content. This is particularly important given contemporary focus on the negative human health outcomes associated with the use of gasoline and diesel fuels.
Some issues, however, arise in the use of methanol in internal combustion engines. Its low vapour pressure and high latent heat of vaporisation cause cold start difficulties at low ambient and in-cylinder temperatures, therefore a glow-plug or ignition enhancer is needed.61 Furthermore, the low lubricity of alcohols, and corrosion susceptibility of metals in alcohols, make fuel additives necessary. Emissions of formaldehyde, acetaldehyde and any unburned methanol are also not sufficiently understood, thus present potential health risks.
In addition, the requirement to carry increased weight will reduce vehicle fuel efficiency.62 A 60 L fuel tank can hold 45 kg of gasoline which can provide approximately 2000 MJ (LHV basis). In contrast, 97 kg of methanol are needed to provide the same energy service. The increased weight of methanol needed to substitute a gasoline-provided energy service will therefore result in a fuel efficiency loss. A study into the effects of oxygenates on vehicle performance observed that they led to an efficiency increase of 5% for a car running on 5% methanol and 95% unleaded gasoline (M5).63 Bardaie and Janius, who investigated the effects of alcohol usage in spark-ignition engines, however observed a power loss of 4–5% for a car running on 100% methanol (M100).64
Of the energy available from the fuel, only about 25% actually gets applied to moving a car (with a gasoline ICE) or running the accessories, the rest is lost to the exhaust gases, coolant, and friction, as illustrated in Fig. 5.65 A reduction in the associated energy losses by ICEs will serve to minimise fuel consumption and improve process efficiency in the future. This conversion ratio has however been assumed for the M100 methanol car in this study (Fig. 6).
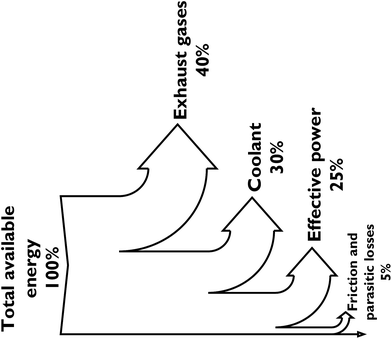 | ||
| Fig. 5 Sankey diagram showing the energy losses associated with converting chemical energy in gasoline to effective power in an internal combustion engine. | ||
On balance, therefore, substituting methanol for gasoline in vehicles will result in a 5% increase in CO2 emissions per energy service delivered.
3.2 Methanol production
Methanol synthesis is a commercially viable but mature process. Some reviews covering the development (potential) of this process have been published over the recent years.66–68 However, since the process is mature, most research activity relating to methanol revolves around methanol fuel cells,69,70 the production of methanol from CO2,71–74 as in this study, the reforming of methanol,75,76 its use in the synthesis of bio-diesel fuel,77,78 its blending properties for fuel blends such as M10,79–81 and its transformation in downstream processes into olefins82,83 and ultimately, gasoline.84,85 China certainly has adopted methanol as a key platform chemical and has become the largest producer – and consumer – of methanol in the world.Methanol can be produced via the catalytic hydrogenation of CO2. There are two main reaction pathways – one in which methanol is directly produced according to eqn (3), and a second in which CO produced via the reverse water gas shift (RWGS) reaction is subsequently hydrogenated to produce CH3OH, according to eqn (4) and (5), respectively.72
 | (3) |
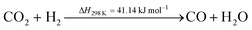 | (4) |
 | (5) |
An appropriate catalyst needs to be chosen to maximise the CO2 yield by enhancing the reaction shown in eqn (3) and inhibiting the reaction represented by eqn (4). This would also serve to reduce the operating costs of the process due to reduced recycle. It has been shown that CO2 and H2 react directly to form CH3OH over commercially-available CuO/ZnO/Al2O3 catalyst without significant production of CO as an intermediate.86
There have been several studies of the production of methanol through catalytic hydrogenation of CO2.54,87–89 The process designed by Rihko-Struckmann et al. makes the same design choices described above, i.e. alkaline water electrolysers and CuO/ZnO/Al2O3 catalyst, and has been used as the reference process for this work. Fresh CO2 and electrolytic hydrogen, which are available at ambient pressure, are compressed to 50 bar before being sent to four adiabatic reactor units in cascade with intercooling. Equilibrium conversion with the defined stoichiometric reactions (eqn (3)–(5)) was assumed. The selected pressure and temperature ranges corresponded to typical industrial conditions for low pressure methanol synthesis.54
3.3 Closing the loop
To avoid the partial decarbonisation scenarios discussed earlier and close the CO2 loop, the CO2 emissions from the methanol-powered cars must be recaptured and reused. A DAC plant is used to concentrate the atmospheric CO2 contributed by the cars to high-purity CO2 feedstock for methanol production by the process described in Section 3.2.4 Power-to-DAC
Many studies have concluded that mitigation options such as CCS can only become economically-viable through a combination of policy measures and technological advancement.90 CCU, by adding commercial value to CO2 emissions, is asserted to be able to overcome these challenges. However, the sub-processes involved in the power-to-fuel process described above (electrolysis, methanol production, transport) incur energy losses across the value chain. In addition, a substantial amount of energy is required to recapture the exhaust CO2 from the atmosphere. Here, the counterfactual of using curtailed wind to directly operate a DAC plant and subsequent storage is considered in order to evaluate the mitigation impact of using renewable energy to operate a DAC process and compare to that of a CCU process.5 Results and discussion
5.1 The UK as a case study
It is estimated that 1277 GWh of wind power was curtailed in the UK in 2015.91 This has been used as the basis for the following analysis. The ESO model implies that, unless iRES deployment is increased to over 50% of peak demand, the availability of curtailed renewable electricity is not likely to significantly increase. A near-term ramping of iRES capacity is unlikely owing to the associated grid stability and reliability issues. Additionally, current plans are to expand the UK's interconnection capacity.92 This expansion will provide further flexibility to the grid and thus minimise curtailment. Should iRES penetration reach the levels required to see surplus electricity availability, the ESO model suggests that this will be for a few hours per day. This means that the various elements of the power-to-fuel and power-to-DAC processes will be required to operate in a flexible manner, and at low capacity factors, posing additional technical and economic challenges. One such challenge will be for the kiln in the DAC plant. Calcination of CaCO3 occurs at 900 °C in the kiln. The repeated cooling and heating of the kiln due to intermittent energy supply will be a source of efficiency loss and may threaten process stability. Therefore, if this course of action is to be pursued, it will be vital to design DAC processes so as to ensure their suitability for this kind of intermittent operation. Furthermore, a plant solely dependent on intermittent energy supply to operate will require labour arrangements that differ from conventional practice.
Table 4 shows the mass and energy balances for the production of a tonne of methanol annually via the power-to-fuel process, assuming 1277 GWh y−1 of renewable electricity remain available and the lower-bound energy consumption of 5.4 GJe tCO2−1 for the DAC technology. The arrow widths are scaled to the amount of mass or energy flow required relative to the water and energy input to the electrolysers, respectively. Due to the dilute nature of CO2 in air, large volumes of air are needed to capture a substantial amount of CO2. To recapture the CO2 emitted by the methanol cars, 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 m3 tCO2−1 and 27
000 m3 tCO2−1 and 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 m3 tCO2−1 of air are required by the lower- and upper-bound DAC technologies considered, which have capture efficiencies of 80% and 50%, respectively.
000 m3 tCO2−1 of air are required by the lower- and upper-bound DAC technologies considered, which have capture efficiencies of 80% and 50%, respectively.
| Hydrogen plant | Methanol plant | Methanol car | Direct air capture plant | |||||
|---|---|---|---|---|---|---|---|---|
| In | Out | In | Out | In | Out | In | Out | |
| Flow rate (t y −1 ) | ||||||||
| H2O | 1.76 | 0.08 | ||||||
| H2 | 0.20 | 0.20 | ||||||
| O2 | 1.57 | 0.48 | ||||||
| CH3OH | 1.00 | 1.00 | ||||||
| CO2 | 1.44 | 0.06 | 1.38 | 1.38 | ||||
| Air | 28.2 | 26.8 | ||||||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||
| Energy (MWh y −1 ) | ||||||||
| Electricity | 10.31 | 1.34 | 2.06 | |||||
| Heat | 3.92 | 0.25 | ||||||
| Effective power | 1.37 | |||||||
| Losses (exhaust, coolant, friction) | 4.10 | |||||||
Approximately 12 MWh tMeOH−1 of electricity was required for methanol production alone, with the bulk of the curtailed electricity – 89% – being consumed by the electrolysis plant. An additional 2.1 MWh tMeOH−1 was required to recapture the CO2 emitted from methanol combustion.
5.2 Mitigation potential
In the UK, gasoline accounts for 33 vol% of transport fuel used in the UK, resulting in the emission of 40 MtCO2 per year to the atmosphere.93 Here, a mitigation potential (MP) has been defined to quantify the prospects of both power-to-fuel and power-to-DAC processes for avoiding GHG emissions. The MP for the power-to-fuel process has been defined as the percentage of gasoline consumption that can be displaced by the produced methanol (see eqn (6)). | (6) |
 | (7) |
Thus, 1277 GWh per year of curtailed renewable energy supports the production of approximately 140 million litres of methanol annually. This is enough to displace 62 million litres of gasoline, thereby giving the power-to-fuel process a MP of 0.37%. Power-to-DAC was found to achieve MP values of 2.1% and 0.7% for the lower- and upper-bound energy costs of capture, respectively. Fig. 7 illustrates the mitigation potential of both processes in terms of number of cars for which CO2 emissions are avoided. A gasoline-powered car is assumed to emit 1.3 tCO2 annually.‡‡ Power-to-DAC can avoid the direct CO2 emissions of 0.24 to 0.67 million cars annually, 2–6 times greater than that achieved by power-to-fuel – 0.12 million cars. While only the emissions from combustion have been taken into account in the above analysis, the same trends have been observed when life cycle analysis (LCA) data is considered.40,96
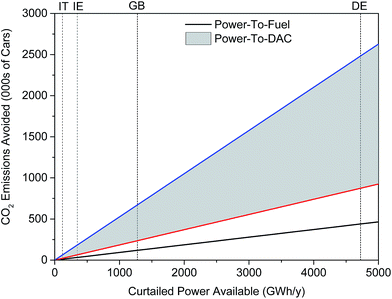 | ||
| Fig. 7 Mitigation potential of both power-to-fuel and power-to-DAC processes in several EU countries: Italy (IT), Ireland (IE), United Kingdom (GB) and Germany (GE). Curtailment figures were obtained from: IT,91 IE,97 GB91 and DE.98 Note that many countries do not differentiate between constraint and curtailment so these figures may be a sum of both. | ||
5.3 Mitigation costs
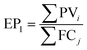 | (8) |
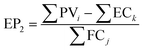 | (9) |
| Chemicals | Prices, $ t−1 |
|---|---|
| a Fossil CO2 is obtained from a coal plant with post-combustion carbon capture and storage. b Non-fossil CO2 is obtained from a DAC plant. | |
| Fossil CO2a | 37–120 (ref. 101) |
| Non-fossil CO2b | 450–550 (ref. 47) |
| Fossil H2 | 1300–2100 (ref. 102 and 103) |
| Electrolytic H2 | 4200 (ref. 104) |
| CH3OH | 450 (ref. 105) |
The commercial viability of methanol production was found to be most sensitive to the hydrogen price. This means that as opposed to focusing on developing improved catalysts to enable the cost effective conversion of CO2 to fuels, it is more important to focus on developing less costly routes to the production of green hydrogen. A study evaluating the economic performance of a plant producing methanol via CO2 hydrogenation with electrolytic hydrogen was found to have a negative margin of $126 million per year.88 This translates to a cost of $209 tCO2−1 avoided.§§ Using this information, a sensitivity analysis was carried out to determine how the profitability of methanol production changes in different feed and product price scenarios. Fig. 8 illustrates the methanol prices needed for the plant to break-even after its assumed 20 year lifetime. It is important to note that intermittent nature of energy supply, which will increase the capital costs of the methanol production plant, was not considered in the cited study.
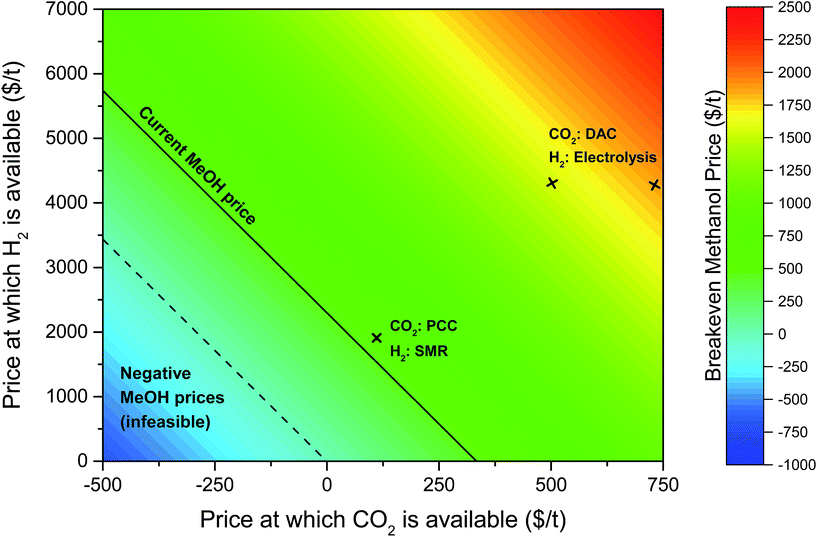 | ||
| Fig. 8 Contour plot showing the variation in the price of methanol needed with hydrogen and carbon dioxide prices for the methanol production plant to breakeven after 20 years. The solid black line indicates the current price of methanol ($450 t−1) and the dashed black line indicates zero methanol price, i.e. the point below which the breakeven price of methanol is negative. The breakeven methanol price when CO2 is sourced from a coal plant with post-combustion capture (PCC)106 and hydrogen from steam methane reforming (SMR) of natural gas102 is also highlighted. The breakeven methanol prices for the power-to-fuel process considered in this study (using CO2via direct air capture (DAC) and electrolytic hydrogen) are also shown above. Note that a range of prices for fossil-derived H2 and CO2 is available in the literature (see Table 5), which will slightly alter the breakeven price of methanol. | ||
At today's hydrogen and carbon dioxide prices (see Table 5), methanol price must more than double to $960 tMeOH−1 for the plant to break even; if carbon dioxide and methanol prices stayed constant, then the hydrogen price would need to reduce by a factor of 2.6 to $1635 tH2−1; if hydrogen and methanol prices stayed constant, a credit of $283 tCO2 avoided−1 would need to be provided. Annotated in Fig. 8 is the methanol price if CO2 and H2 were sourced from cheaper alternative processes, at costs equivalent to CO2 from a post-combustion capture (PCC) from a power plant and H2 from the steam reforming of methane (SMR). This would still require a methanol price of $523 tMeOH−1, 16% higher than current prices. It is important to note that both of these alternatives involve fossil fuel use, hence are contrary to our objective of maximising the mitigation potential of renewable electricity.
Direct air capture. The 2011 APS report presented a techno-economic assessment for a proposed DAC plant capturing 1 MtCO2 per year. The cost of capture was evaluated to be $430–550 tCO2−1.47 The cost per tonne of CO2 avoided, Cavoided, can be evaluated using eqn (10) below.
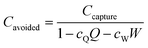 | (10) |
5.4 Process improvements
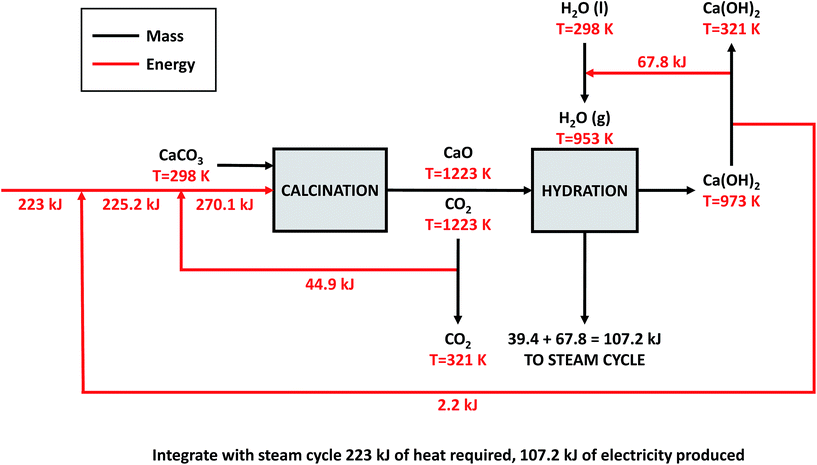 | ||
| Fig. 11 Proposed heat integration for the direct air capture process proposed in the 2011 APS report. | ||
Conventional cryogenic air separation consumes 245 kW h tO2−1 produced.107 Co-location of the electrolysis and DAC plants could therefore provide energy savings in the range 17–34 GWh y−1 for the lower and upper bound DAC technologies considered, respectively, equivalent to 6% of the annual primary energy consumption by the DAC plants. ASU costs of $10–100 tO2−1 have been cited in the literature108 so co-locating the plants can drive down mitigation costs by $5–48 tCO2−1.
Direct air capture. The heat required to calcine CaCO3 is required to be at a temperature greater than ∼1223 K, practically necessitating the combustion of a fuel (and this must be done using an oxy-fired system to produce a pure stream of CO2). Usual practice in the calcium looping cycle for CO2 capture from a power station would be to integrate a steam cycle at around 973 K. This very significantly enhances the process efficiency. However, in the cycle proposed for CO2 capture from the air, the exothermic CO2 capture stage occurs at ambient temperature, effectively slightly increasing the air temperature, but wasting the opportunity to integrate heat, effectively throwing away 100% of the high grade heat. The opportunity exists to potentially operate a steam cycle to reclaim some of the hydration heat, but this requires pressurisation of the hydrator – see Appendix 1 for details. Heat integration could potentially reduce the primary energy demand of the upper-bound DAC plant to 8.79 GJ tCO2−1 captured. This could save 157 TJ y−1, or approximately 30% of the natural gas requirement of the plant. The reconversion efficiency of the power-to-fuel process is therefore increased from 33.3% to 36.5% (η is increased from 8.3% to 9.1% if power-to-mobility is considered).
Commercial pursuit of DAC technology has largely focused on modular units that allow for ease of scale-up.41–43 This, potentially, creates scope for cost reduction through mass production. As DAC technology is common to both the power-to-fuel and the power-to-DAC processes discussed in this study, their relative costs will be independent of the actual cost of DAC.
6 Conclusions
CCU is often proposed as a means improve the economic viability of CO2 mitigation. To reduce the associated life-cycle emissions of CO2-based fuels compared to fossil fuels, the material and energy inputs typically need to be derived from renewable sources. CCU processes often aim to use curtailed renewable electricity. As the UK electricity system transforms to meet its decarbonisation target, this study finds that material levels of curtailment are unlikely at iRES penetration levels of <50%, which are unlikely before 2035. Furthermore, whilst it is evident that instances of surplus renewable energy have existed in different regions, measures being pursued, including the expansion of regional transmission networks, will serve to keep curtailment below contemporary levels. The curtailed power is unlikely to be available at zero cost, as evidenced by the constraint payments being made to UK wind farms to curtail their generation. Therefore, arguments basing CCU process operations only on the use of curtailed iRES are not likely.However, where curtailed renewable energy does exist, direct removal of CO2 from the atmosphere via direct air capture (DAC) can mitigate 2–6 times more CO2 than converting CO2 into methanol, per unit of available energy. On the basis of 1277 GWh y−1 electricity availability, DAC can avoid 0.7–2.1% of the UK's 2016 gasoline-derived emissions at a cost of $430–660 tCO2 avoided−1. Using this electricity for methanol production and subsequent recycling of CO2, however, can avoid 0.37% of gasoline-derived emissions at a cost of $640–870 tCO2 avoided−1. The additional costs incurred by methanol production are largely due to the cost of H2 feedstock which significantly outweigh the revenue from methanol sale. We find that in terms of mitigation potential and costs, power-to-DAC is better than power-to-fuel in all scenarios. Furthermore, low-pressure methanol synthesis is a commercially-viable and mature process.66–68,109 DAC technology, however, is still a novel technology with current RD&D efforts geared towards developing modular systems, providing promise for cost reduction and scalability. Both power-to-fuel and power-to-DAC processes would benefit from better DAC systems.
Appendix 2 discusses the opportunities for advancement in methanol synthesis technology and catalysts. It was found that even if the catalyst is improved to further facilitate methanol production, the costs of non-fossil CO2 and electrolytic hydrogen must reduce significantly for the power-to-fuel process to be economically feasible.
A core conclusion of this study is that using surplus renewable energy to capture CO2 directly from the atmosphere via DAC is a superior option, both from an environmental and an economic point of view, to using it to produce methanol for use as a gasoline substitute. Thus, more CO2 emissions would be avoided if fossil fuels are further burned and renewable energy is used for DAC than by converting the captured CO2 into fuels.
This study has focused on the maximum climate change mitigation that is achievable for a given supply of surplus electricity. Should curtailed electricity be used, the electrolysis, methanol and DAC plants must be designed for intermittent operation, i.e. at low load factors. Consequently, the labour arrangements will differ from conventional practice in chemical plants. This could have significant implications on costs. The analysis in Fig. 8 illustrates the effects of increased CO2 and H2 prices on the breakeven price of methanol, which determines the financial viability of the process. Our analysis neglected the effects of intermittency and thus presents an optimistic scenario. To quantify the impact of intermittent operation on capital and operating costs, detailed design of the individual processes is needed, which is beyond the scope of this current study. Since intermittency would affect both power-to-methanol and power-to-DAC plant, we believe that main findings of the comparison of both routes would still remain valid given a more detailed analysis.
7 Implications for policymakers
There are two primary conclusions to this study. First, in any well-designed and professionally operated electricity system, the spectre of significant quantities of curtailed renewable energy is unlikely to appear in the medium to long term. For this reason, arguments to support initiatives aimed at utilising this curtailed renewable energy should be viewed with caution. Second, in order to avoid lock-in to partial decarbonisation scenarios, any fossil carbon which is extracted from the geosphere must be promptly returned to the geosphere. In net-zero and net-negative emission scenarios, processes which seek to convert carbon to short-lived products – essentially all CO2 utilisation options aside from mineral carbonation – will need to be coupled with direct air capture or use biomass feedstock to recapture the CO2 arising from these processes. This has the consequence that on a technical, economic and climate change mitigation basis, direct air capture and subsequent CO2 storage would appear to offer superior value than the conversion of CO2. Still, policymakers and societies might opt for the less efficient route via conversion of CO2 due to public acceptance, or increased energy security. The present study quantifies the corresponding significant increase in cost and loss in mitigation efficiency which have to be taken into account.Appendix 1
The DAC design presented in the APS report has simply considered the energy requirement for heating CaCO3 from 298 K to around 1223 K, followed by calcination, to achieve the figure of 6.1 GJ tCO2 captured−1, with an appropriate kiln efficiency then assumed on top of this figure (to yield a total of 6.1/ηkiln = 8.13 GJ tCO2−1 primary energy requirement). Considering the flowsheet in Fig. 1 carefully, there is the possibility of reclaiming some heat from the exothermic hydration reaction described in eqn (12). This could be either used to preheat the CaCO3 entering the kiln or alternatively used to raise steam to offset some of the on-site electricity costs. Prior to heat integration, the main reactions are eqn (11) and (12), calcination and hydration (including relevant temperature changes). To obtain heat at a sufficiently high temperature for either effective preheating or electricity generation, the hydration reactor would have to be pressurized. Here, it has been assumed that the pressure is maintained at 16.3 bara, corresponding to the equilibrium pressure for the reaction between CaO and H2O at 973 K. CaO(s) is presumed to be produced at 1223 K, and H2O(l) is available at ambient temperature, 298 K. All heat exchangers have been assumed to require 20 K temperature difference.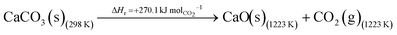 | (11) |
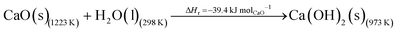 | (12) |
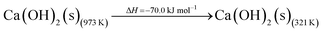 | (13) |
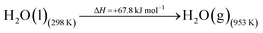 | (14) |
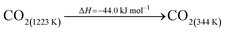 | (15) |
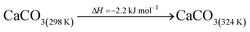 | (16) |
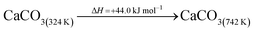 | (17) |
High temperature calcination (eqn (11)) comprises majority of the heat requirement of the system. Heat is available at 973 K via reaction 12, and from the cooling of Ca(OH)2 from reaction temperature to near ambient temperature, eqn (13). The heat liberated in reaction (14) can be used to vapourise and preheat water viaeqn (16), with the potential for a small preheat of CaCO3 with any remaining heat from eqn (16). This would effectively increase the energy available to produce electricity via reaction (12), and very slightly decrease the heat requirement for reaction (11). There is of course heat available from cooling down the hot CO2, which can be used to further raise the temperature of the CaCO3 which was preheated under eqn (16)viaeqn (17). Failure to integrate with a steam cycle severely limits the heat integration possibilities of the process.
The scheme above yields a heat requirement of 223.9 kJ and yields 107.2 kJ of energy to be taken out in a steam cycle. This is equivalent to 5.08 GJth tCO2−1 (6.78 GJ tCO2−1 of primary energy after taking into account kiln efficiency) and with electricity assumed to be produced from the steam cycle with an efficiency of 40%, yielding 0.97 GJe tCO2−1, with a net import 0.81 GJe tCO2−1, requiring 2.01 GJ tCO2−1 of natural gas to produce. Overall, this yields a net heat requirement of 8.79 GJ tCO2−1. The scheme described here is, as far as we can see, the most heat integrated method possible (and does not take into account capital costs, which may well be very large). It is not proposed to consider heat transfer within the KOH part of the process, since heat recovery from the aqueous solutions will be challenging, the heat of reaction for the CO2 transfer from potassium to carbonate is small, and the large air flows will continually cool the circulating solution to ambient temperature.
If the primary energy requirement of the DAC processes presented in the APS report47 (upper-bound DAC plant) is reduced to 8.79 GJ tCO2 captured−1via the heat integration presented above, 157 TJ y−1 could be saved. This is equivalent to 30.2% of the natural gas requirement of the plant. The reconversion efficiency of the power-to-fuel process is therefore increased from 33.3% to 36.5% (η is increased from 8.3% to 9.1% if power-to-mobility is considered).
Appendix 2
Research priorities
The catalytic holy grail of methanol synthesis is, of course, the direct partial oxidation of methane to methanol.116 Research has covered high-temperature systems, which are unlikely to become commercially viable as it is difficult to exert sufficient control in radical reactions to afford the required selectivity at appreciable conversion.117,118 Low temperature synthesis has been demonstrated with highly acidic systems that can stabilise methyl groups.119 Industrially this would require costly materials to deal with the acid nature of the process. Routinely, biological systems perform this reaction at high turnover frequencies and room temperature. The enzyme (catalyst) facilitating this action is methanol mono-oxygenase (MMA).120 Inorganic equivalents bases on Cu and Fe exchanged zeolites have shown significant activity and selectivity.121,122 However, selectivity and yields need to be improved significantly to give this approach industrial relevance.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank the “Science and Solutions for a Changing Planet Doctoral Training Programme” (SSCP DTP) by the Natural Environment Research Council (NERC), the Department of Chemical Engineering at Imperial College London, the IEA Greenhouse Gas R&D Programme (IEAGHG) and the MESMERISE-CCS project under grant EP/M001369/1 from the Engineering and Physical Sciences Research Council (EPSRC) for the funding of PhD scholarships and support of this project. The authors are grateful to Dr J. Yao for his help in checking the calculations presented in Appendix 1. André Bardow acknowledges that part of this work was supported by the Cluster of Excellence “Tailor-Made Fuels from Biomass”, which is funded under contract EXC 236 by the Excellence Initiative by the German federal and state governments to promote science and research at German universities.References
- IPCC, Climate Change 2014: Mitigation of Climate Change, Cambridge University Press, Cambridge, United Kingdom, 2014 Search PubMed.
- G. Lomax, T. M. Lenton, A. Adeosun and M. Workman, Nat. Clim. Change, 2015, 5, 498–500 CrossRef.
- E. Kriegler, O. Edenhofer, L. Reuster, G. Luderer and D. Klein, Clim. Change, 2013, 118, 45–57 CrossRef CAS.
- P. Smith, S. J. Davis, F. Creutzig, S. Fuss, J. Minx, B. Gabrielle, E. Kato, R. B. Jackson, A. Cowie, E. Kriegler, D. P. van Vuuren, J. Rogelj, P. Ciais, J. Milne, J. G. Canadell, D. McCollum, G. Peters, R. Andrew, V. Krey, G. Shrestha, P. Friedlingstein, T. Gasser, A. Grübler, W. K. Heidug, M. Jonas, C. D. Jones, F. Kraxner, E. Littleton, J. Lowe, J. R. Moreira, N. Nakicenovic, M. Obersteiner, A. Patwardhan, M. Rogner, E. Rubin, A. Sharifi, A. Torvanger, Y. Yamagata, J. Edmonds and C. Yongsung, Nat. Clim. Change, 2015, 6, 42–50 CrossRef.
- M. Fajardy and N. Mac Dowell, Energy Environ. Sci., 2017, 10, 1389–1426 CAS.
- P. Psarras, H. Krutka, M. Fajardy, Z. Zhang, S. Liguori, N. M. Dowell and J. Wilcox, Wiley Interdiscip. Rev.: Energy Environ., 2017, 6, e253 CrossRef.
- I. Dimitriou, P. Garcia-Gutierrez, R. H. Elder, R. M. Cuellar-Franca, A. Azapagic and R. W. K. Allen, Energy Environ. Sci., 2015, 8, 1775–1789 CAS.
- M. E. Boot-Handford, J. C. Abanades, E. J. Anthony, M. J. Blunt, S. Brandani, N. Mac Dowell, J. R. Fernandez, M.-C. Ferrari, R. Gross, J. P. Hallett, R. S. Haszeldine, P. Heptonstall, A. Lyngfelt, Z. Makuch, E. Mangano, R. T. J. Porter, M. Pourkashanian, G. T. Rochelle, N. Shah, J. G. Yao and P. S. Fennell, Energy Environ. Sci., 2014, 7, 130–189 CAS.
- J. Klankermayer, S. Wesselbaum, K. Beydoun and W. Leitner, Angew. Chem., Int. Ed., 2016, 55, 7296–7343 CrossRef CAS PubMed.
- P. Markewitz, W. Kuckshinrichs, W. Leitner, J. Linssen, P. Zapp, R. Bongartz, A. Schreiber and T. E. Muller, Energy Environ. Sci., 2012, 5, 7281–7305 CAS.
- N. Mac Dowell, P. S. Fennell, N. Shah and G. C. Maitland, Nat. Clim. Change, 2017, 243–249 CrossRef CAS.
- J. G. Olivier, G. Janssens-Maenhout, M. Muntean and J. A. Peters, Trends in global CO2 emissions: 2015 Report, PBL Netherlands Environmental Assessment Agency, 2015 Search PubMed.
- N. von der Assen and A. Bardow, Green Chem., 2014, 16, 3272–3280 RSC.
- U.S. Energy Information Aministration, Short-Term Energy Outlook (STEO), 2017 Search PubMed.
- G. Wilson, M. Trusler, J. Yao, J.-S. M. Lee, R. Graham, N. Mac Dowell, R. Cuellar-Franca, G. Dowson, P. Fennell, P. Styring, J. Gibbins, M. Mazzotti, S. Brandani, C. Muller and R. Hubble, Faraday Discuss., 2016, 192, 561–579 RSC.
- J. Petinrin and M. Shaaban, Renewable Sustainable Energy Rev., 2016, 65, 770–783 CrossRef.
- C. F. Heuberger, I. Staffell, N. Shah and N. Mac Dowell, Energy Environ. Sci., 2016, 9, 2497–2510 CAS.
- L. Bird, D. Lew, M. Milligan, E. M. Carlini, A. Estanquiero, D. Flynn, E. Gómez-Lázaro, H. Holtinnen, N. Menemenlis, A. Orths, P. B. Eriksen, J. C. Smith, L. Soder, P. Sorensun, A. Altiparmakis, Y. Yasuda and J. Miller, Renewable Sustainable Energy Rev., 2016, 65, 577–586 CrossRef.
- Y. Scholz, H. C. Gils and R. Pietzcker, Energy Econ., 2016, 64, 568–582 CrossRef.
- National Grid, Monthly Balancing Services Summary 2014/15, 2015 Search PubMed.
- Y. Yasuda, L. Bird, E. M. Carlini, A. Estanqueiro, D. Flynn, A. Forcione, E. Gómez-Lázaro, P. Higgins, H. Holttinen, D. Lew, S. Martin-Martinez, J. McCann, N. Menemenlis and J. C. Smith, Proceedings of WIW2015 Workshop, 2015 Search PubMed.
- P. Heptonstall, R. Gross and F. Steiner, The costs and impacts of intermittency – 2016 update, 2017 Search PubMed.
- C. F. Heuberger, I. Staffell, N. Shah and N. M. Dowell, Comput. Chem. Eng., 2017, 107, 247–256 CrossRef CAS.
- A. Energiewende, Die Energiewende im Stromsektor: Stand der Dinge 2016. Rückblick auf die wesentlichen Entwicklungen sowie Ausblick auf 2017, 2016 Search PubMed.
- Renewable Energy Foundation (REF), Wind Farm Constraint Payments over Easter 2016, 2016 Search PubMed.
- European Wind Energy Association (EWEA), Transmission system operation with a large penetration of wind and other renewable electricity sources in electricity networks using innovative tools and integrated energy solutions (TWENTIES): Final report, 2013 Search PubMed.
- E. V. Mc Garrigle, J. P. Deane and P. G. Leahy, Renewable Energy, 2013, 55, 544–553 CrossRef.
- R. Golden and B. Paulos, Electr. J., 2015, 28, 36–50 CrossRef.
- M. Rothleder, Renewable Integration, California Energy Commission IEPR Workshop, 2017 Search PubMed.
- N. Haga, V. Kortela and A. Ahnger, Reducing Wind Power Curtailment in China, WÄRTSILÄ White Paper, 2016 Search PubMed.
- Ea Energy Analyses, The Danish Experience with Integrating Variable Renewable Energy, Study on behalf of Agora Energiewende, 2015 Search PubMed.
- Department of Energy & Climate Change, Updated energy and emissions projections 2015, 2015 Search PubMed.
- C. F. Heuberger, E. Rubin, I. Staffell, N. Shah and N. M. Dowell, Appl. Energy, 2017, 204, 831–845 CrossRef.
- S. Pfenninger and I. Staffell, Renewables.ninja, 2016, https://beta.renewables.ninja/, accessed: 2017-07-05 Search PubMed.
- I. Staffell and S. Pfenninger, Energy, 2016, 114, 1224–1239 CrossRef.
- S. Pfenninger and I. Staffell, Energy, 2016, 114, 1251–1265 CrossRef.
- National Grid, Monthly Balancing Services Summary 2016, 2016 Search PubMed.
- UK Department of Business, Energy and Industrial Strategy (BEIS), Updated Energy and Emissions Projections 2016, 2017 Search PubMed.
- Committee on Climate Change, Sectoral scenarios for the Fifth Carbon Budget, 2015 Search PubMed.
- A. Sternberg and A. Bardow, Energy Environ. Sci., 2015, 8, 389–400 CAS.
- Climeworks AG, Climeworks CO2 Capture Demonstrator, 2016 Search PubMed.
- Carbon Engineering Ltd, Our Technology, 2015 Search PubMed.
- Global Thermostat Ltd, About Us: A Unique Capture Process, 2010 Search PubMed.
- M. Ranjan and H. J. Herzog, Energy Procedia, 2011, 4, 2869–2876 CrossRef CAS.
- R. Baciocchi, G. Storti and M. Mazzotti, Chem. Eng. Process., 2006, 45, 1047–1058 CrossRef CAS.
- K. S. Lackner, Sci. Am., 2010, 302, 66–71 CrossRef CAS PubMed.
- R. Socolow, M. Desmond, R. Aines, J. Blackstock, O. Bolland, T. Kaarsberg, N. Lewis, M. Mazzotti, A. Pfeffer, K. Sawyer, J. Siirola, B. Smit and J. Wilcox, Direct Air Capture of CO2 with Chemicals. A Technology Assessment for the APS Panel on Public Affairs, 2011 Search PubMed.
- D. W. Keith, M. Ha-Duong and J. K. Stolaroff, Clim. Change, 2006, 74, 17–45 CrossRef CAS.
- M. Carmo, D. L. Fritz, J. Mergel and D. Stolten, Int. J. Hydrogen Energy, 2013, 38, 4901–4934 CrossRef CAS.
- M. Laguna-Bercero, J. Power Sources, 2012, 203, 4–16 CrossRef CAS.
- J. G. Tom Smolinka and M. Günther, Stand und Entwicklungspotenzial der Wasserelektrolyse zur Herstellung von Wasserstoff aus regenerativen Energien, 2011 Search PubMed.
- V. Schröder, B. Emonts, H. Janßen and H.-P. Schulze, Chem. Eng. Technol., 2004, 27, 847–851 CrossRef.
- NEL Hydrogen, Efficient Electrolysers for Hydrogen Production, https://wpstatic.idium.no/www.nel-hydrogen.com/2015/03/Efficient_Electrolysers_for_Hydrogen_Production.pdf, 2016 Search PubMed.
- L. K. Rihko-Struckmann, A. Peschel, R. Hanke-Rauschenbach and K. Sundmacher, Ind. Eng. Chem. Res., 2010, 49, 11073–11078 CrossRef CAS.
- F. Asinger, in Methanol — Chemie- und Energierohstoff, Springer, Berlin Heidelberg, 1986, pp. 1–9 Search PubMed.
- G. A. Olah, Angew. Chem., Int. Ed., 2005, 44, 2636–2639 CrossRef CAS PubMed.
- G. A. Olah, A. Goeppert and G. K. S. Prakash, J. Org. Chem., 2009, 74, 487–498 CrossRef CAS PubMed.
- M. B. Çelik, B. Özdalyan and F. Alkan, Fuel, 2011, 90, 1591–1598 CrossRef.
- M. Eyidogan, A. N. Ozsezen, M. Canakci and A. Turkcan, Fuel, 2010, 89, 2713–2720 CrossRef CAS.
- R. L. Bechtold, Alternative Fuels Guidebook: Properties, Storage, Dispensing, and Vehicle Facility Modifications [R-180], Society of Automotive Engineers Inc., 2014 Search PubMed.
- P. Roy, R. Roy and B. K. Mandal, Int. J. Sci. Eng. Res., 2013, 4(12), 986–989 Search PubMed.
- Ricardo Inc., Impact of Vehicle Weight Reduction on Fuel Economy for Various Vehicle Architectures, 2008 Search PubMed.
- F. H. Palmer, Vehicle Performance of Gasoline Containing Oxygenates. International Conference on Petroleum Based Fuels and Automotive Applications (IMechE Conference Publications 1986-11), Society of Automotive Engineers Inc, 1986 Search PubMed.
- M. Z. Bardaie and R. Janius, Ama, Agric. Mech. Asia, Afr. Lat. Am., 1984, 31–34 Search PubMed.
- Green Car Congress, DOE Co-Funds 12 Projects to Increase Engine Efficiency, 2005 Search PubMed.
- J.-P. Lange, Catal. Today, 2001, 64, 3–8 CrossRef CAS.
- K. A. Ali, A. Z. Abdullah and A. R. Mohamed, Renewable Sustainable Energy Rev., 2015, 44, 508–518 CrossRef CAS.
- A. Riaz, G. Zahedi and J. J. Kleme
![[s with combining breve]](https://www.rsc.org/images/entities/char_0073_0306.gif) , J. Cleaner Prod., 2013, 57, 19–37 CrossRef CAS.
, J. Cleaner Prod., 2013, 57, 19–37 CrossRef CAS. - S. Wasmus and A. Küver, J. Electroanal. Chem., 1999, 461, 14–31 CrossRef CAS.
- N. Radenahmad, A. Afif, P. I. Petra, S. M. Rahman, S.-G. Eriksson and A. K. Azad, Renewable Sustainable Energy Rev., 2016, 57, 1347–1358 CrossRef CAS.
- I. Ganesh, Renewable Sustainable Energy Rev., 2014, 31, 221–257 CrossRef CAS.
- S. G. Jadhav, P. D. Vaidya, B. M. Bhanage and J. B. Joshi, Chem. Eng. Res. Des., 2014, 92, 2557–2567 CrossRef CAS.
- W.-H. Wang, Y. Himeda, J. T. Muckerman, G. F. Manbeck and E. Fujita, Chem. Rev., 2015, 115, 12936–12973 CrossRef CAS PubMed.
- K. Li, X. An, K. H. Park, M. Khraisheh and J. Tang, Catal. Today, 2014, 224, 3–12 CrossRef CAS.
- A. Iulianelli, P. Ribeirinha, A. Mendes and A. Basile, Renewable Sustainable Energy Rev., 2014, 29, 355–368 CrossRef CAS.
- S. Yong, C. Ooi, S. Chai and X. Wu, Int. J. Hydrogen Energy, 2013, 38, 9541–9552 CrossRef CAS.
- E. Aransiola, T. Ojumu, O. Oyekola, T. Madzimbamuto and D. Ikhu-Omoregbe, Biomass Bioenergy, 2014, 61, 276–297 CrossRef CAS.
- B. Bharathiraja, M. Chakravarthy, R. R. Kumar, D. Yuvaraj, J. Jayamuthunagai, R. P. Kumar and S. Palani, Renewable Sustainable Energy Rev., 2014, 38, 368–382 CrossRef CAS.
- X. Zhen and Y. Wang, Renewable Sustainable Energy Rev., 2015, 52, 477–493 CrossRef CAS.
- A. Elfasakhany, Int. J. Eng. Sci. Technol., 2015, 18, 713–719 CrossRef.
- I. Yusri, R. Mamat, G. Najafi, A. Razman, O. I. Awad, W. Azmi, W. Ishak and A. Shaiful, Renewable Sustainable Energy Rev., 2017, 77, 169–181 CrossRef CAS.
- P. Tian, Y. Wei, M. Ye and Z. Liu, ACS Catal., 2015, 5, 1922–1938 CrossRef CAS.
- K. Hemelsoet, J. Van der Mynsbrugge, K. De Wispelaere, M. Waroquier and V. Van Speybroeck, ChemPhysChem, 2013, 14, 1526–1545 CrossRef CAS PubMed.
- A. Galadima and O. Muraza, J. Nat. Gas Sci. Eng., 2015, 25, 303–316 CrossRef CAS.
- Z. Wan, W. Wu, W. Chen, H. Yang and D. Zhang, Ind. Eng. Chem. Res., 2014, 53, 19471–19478 CrossRef CAS.
- M. Saito, T. Fujitani, M. Takeuchi and T. Watanabe, Appl. Catal., A, 1996, 138, 311–318 CrossRef CAS.
- É. S. Van-Dal and C. Bouallou, J. Cleaner Prod., 2013, 57, 38–45 CrossRef.
- M. Pérez-Fortes, J. C. Schöneberger, A. Boulamanti and E. Tzimas, Appl. Energy, 2016, 161, 718–732 CrossRef.
- D. Milani, R. Khalilpour, G. Zahedi and A. Abbas, J. CO2 Util., 2015, 10, 12–22 CrossRef CAS.
- The Secretary of Energy Advisory Board (SEAB) CO2 Utilization Task Force, Task Force on RD&D strategy for CO2 Utilization and/or Negative Emissions at the gigatonne scale, U.S. Department of Energy technical report, 2016 Search PubMed.
- WindEurope, WindEurope views on curtailment of wind power and its link to priority dispatch, 2016 Search PubMed.
- Office of Gas and Electricity Markets, Electricity interconnectors, https://www.ofgem.gov.uk/electricity/transmission-networks/electricity-interconnectors, 2017 Search PubMed.
- Department for Transport, Table ENV0501. Volume of fuels by fuel type: United Kingdom, 2016 Search PubMed.
- Department for Transport, Table VEH0150. Vehicles registered for the first time by body type, Great Britain, monthly: January 2001 to June 2015, Vehicle Licensing Statistics, 2015 Search PubMed.
- Department for Transport, Table NTS0901. Annual mileage of 4-wheeled cars by ownership and trip purpose: England, 2002 to 2016, National Travel Survey 2016, 2016 Search PubMed.
- J. Artz, T. E. Müller, K. Thenert, J. Kleinekorte, R. Meys, A. Sternberg, A. Bardow and W. Leitner, Chem. Rev., 2017, 118(2), 434–504 CrossRef PubMed.
- EIRGRID and SONI, Annual Renewable Energy Constraint and Curtailment Report 2015, 2016 Search PubMed.
- Bundesnetzagentur, EEG in Zahlen 2015-Inhaltsverzeichnis, 2016 Search PubMed.
- J. M. Douglas, Conceptual Design of Chemical Processes, McGraw-Hill, 1988 Search PubMed.
- U.S. Energy Information Administration, Levelised Cost and Levelized Avoided Cost of New Generation Resources in the Annual Energy Outlook 2016, 2016 Search PubMed.
- E. S. Rubin, J. E. Davison and H. J. Herzog, Int. J. Greenhouse Gas Control, 2015, 40, 378–400 CrossRef CAS.
- B. Bonner, Current Hydrogen Cost, DOE Hydrogen and Fuel Cell Technical Advisory Committee, 2013, https://www.hydrogen.energy.gov/pdfs/htac_oct13_10_bonner.pdf, accessed on 26-01-2018 Search PubMed.
- G. Collodi, G. Azzaro, N. Ferrari and S. Santos, Energy Procedia, 2017, 114, 2690–2712 CrossRef CAS.
- Office of Energy Efficiency and Renewable Energy, Multi-Year Research, Development, and Demonstration Plan, 2015 Search PubMed.
- Methanex, Methanex posts regional contract methanol prices for North America, Europe and Asia, 2017 Search PubMed.
- Global Carbon Capture and Storage Institute Ltd, The Costs of CCS and Other Low-Carbon Technologies in the United States - 2015 Update, 2015 Search PubMed.
- Gerhard Beysel - The Linde Group, 1st Oxyfuel Combustion Conference, 2009 Search PubMed.
- A. Ebrahimi, M. Meratizaman, H. A. Reyhani, O. Pourali and M. Amidpour, Energy, 2015, 90, 1298–1316 CrossRef CAS.
- I. Sharafutdinov, S. Dahl and I. Chorkendorff, Ph.D. thesis, Department of Physics, Technical University of Denmark, 2013.
- A. Cybulski, Catal. Rev., 1994, 36, 557–615 CAS.
- N. J. Brown, J. Weiner, K. Hellgardt, M. S. P. Shaffer and C. K. Williams, Chem. Commun., 2013, 49, 11074–11076 RSC.
- B. Li and K.-J. Jens, Ind. Eng. Chem. Res., 2014, 53, 1735–1740 CrossRef CAS.
- A. Bansode and A. Urakawa, J. Catal., 2014, 309, 66–70 CrossRef CAS.
- A. Álvarez, A. Bansode, A. Urakawa, A. V. Bavykina, T. A. Wezendonk, M. Makkee, J. Gascon and F. Kapteijn, Chem. Rev., 2017, 117, 9804–9838 CrossRef PubMed.
- N. von der Assen, J. Jung and A. Bardow, Energy Environ. Sci., 2013, 6, 2721–2734 CAS.
- V. Arutyunov, Direct Methane to Methanol, Elsevier Science & Technology, 2014 Search PubMed.
- A. Holmen, Catal. Today, 2009, 142, 2–8 CrossRef CAS.
- M. Alvarez-Galvan, N. Mota, M. Ojeda, S. Rojas, R. Navarro and J. Fierro, Catal. Today, 2011, 171, 15–23 CrossRef CAS.
- R. A. Periana, D. J. Taube, E. R. Evitt, D. G. Löffler, P. R. Wentrcek, G. Voss and T. Masuda, Science, 1993, 259, 340–343 CAS.
- J. Liao, L. Mi, S. Pontrelli and S. Luo, Nat. Rev. Microbiol., 2016, 14, 288–304 CrossRef CAS PubMed.
- B. Michalkiewicz, Appl. Catal., A, 2004, 277, 147–153 CrossRef CAS.
- C. Kalamaras, D. Palomas, R. Bos, A. Horton, M. Crimmin and K. Hellgardt, Catal. Lett., 2016, 146, 483–492 CrossRef CAS.
Footnotes |
| † Currently visiting the Department of Chemical and Biological Engineering, University of British Columbia, Vancouver, Canada. |
| ‡ These authors supervised the analysis. |
| § Available power not generated or not supplied to transmission grid. |
| ¶ System Non-Synchronous Penetration (SNSP) is the ratio of non-synchronous generation (wind and HVDC imports) to demand including HVDC exports. This limit is set to 50% in the Irish power grid in order to maintain grid stability and operability. |
| || Using March 2016 exchange rate of $1.4318 per £. |
| ** In Germany, negative costs are currently paid due to policies enforcing the grid operations to feed in renewables. However, the hours with negative costs have been reducing in 2016 as well as the average negative cost.24 |
| †† The process described in the APS report (see Table 1) requires both heat and electricity. For the purpose of this study, an electricity-to-heat conversion efficiency of 45% has been taken into account. |
| ‡‡ Based on average CO2 emissions and annual mileage of 121.3 gCO2 km−1 (ref. 94) and 6500 miles,95 respectively, for gasoline-powered cars in England. |
| §§ Assuming 2014 exchange rate of 1.3285$ per €. |
| ¶¶ Properties of methane have been assumed for natural gas (HHV of 55.5 MJ kg−1 and complete combustion producing 2.75 kgCO2 kgNG−1). |
| This journal is © The Royal Society of Chemistry 2018 |