High energy and high voltage integrated photo-electrochemical double layer capacitor†
Abstract
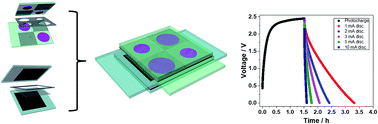
A novel, monolithic harvesting–storage (HS) device composed of a dye-sensitized solar cell (DSSC)-based module and a high voltage supercapacitor with impressive discharge capacity after photocharging is herein proposed. Both the harvesting and the storage sections are fabricated onto conductive glass substrates, paving the way to a smart and easy integration in window facades for energy self-sustainable buildings. In addition, the HS device can also be integrated in portable electronics or drive remote, off-grid sensor networks requiring high power intermittent electrical energy. The harvesting photovoltaic section is constituted by a series of four DSSCs integrated in a single W-type module while the storage section consists of an activated carbon-based supercapacitor (SC) utilizing Pyr14TFSI ionic liquid as the electrolyte. The testing of the two separated sections as well as of the integrated system is reported here. In particular, the integration is evaluated through photo-charge and subsequent discharge protocols performed at different galvanostatic currents, showing that the SC handles photo-charges up to 2.45 V while delivering discharge capacities exceeding 1.8 mA h (0.1 mA h cm−2) upon 1 mA discharge current. To the best of our knowledge this is a never reported before, absolute record value, for stable and reliable integrated HS devices.
- This article is part of the themed collection: 2018 Sustainable Energy and Fuels HOT Articles


 Please wait while we load your content...
Please wait while we load your content...