Advances in in situ and ex situ tar reforming with biochar catalysts for clean energy production
Received
14th November 2017
, Accepted 15th December 2017
First published on 18th December 2017
Abstract
Thermochemical processes such as gasification can decompose carbon feedstocks into gaseous (e.g., syngas), liquid (e.g., oil, tar), and solid (e.g., char) products. Gasification is generally performed in a partial-oxidation environment (e.g., CO2 and steam). In particular, steam gasification has attracted more attention for converting biomass into valuable H2-rich syngas. Biochar is the predominant by-product from biomass pyrolysis or gasification, which has high potential to be used for gas cleaning (e.g., tar removal). This paper reviewed the progress in tar cracking/reforming with biochar catalysts. Biochar can be used as a carbon catalyst or support with fair performance in tar removal. It is noteworthy that biochar catalysts can also be directly gasified to recover energy without the need for frequent regeneration after deactivation. Besides, an integrated strategy of catalytic biomass pyrolysis was proposed to promote the environmentally friendly application of biomass gasification in the future. In general, the high catalytic activity of biochar for tar reforming is attributed to its specific surface properties, amorphous carbon structure with active surface functional groups, and the properties of metal species (e.g., concentration, physicochemical state and dispersion) such as AAEMs in the biochar matrix. Particularly, K-loaded char is significant for tar decomposition in H2O or CO2.
1. Introduction
Biomass is a renewable energy resource derived from biological sources, such as energy crops, agricultural residues, forestry residues and algae. Biomass utilization is recognized as one of the most promising solutions for current energy and environmental problems. Alternative renewable energy technologies such as hydropower energy, solar energy and wind power, which often suffer from the intermittent power generation issue, are less reliable for the security of supply. Meanwhile, biomass is the only renewable energy source that can be converted into liquid fuels and used as feedstock in chemical synthesis.1
Thermochemical processes including combustion, pyrolysis and gasification can convert biomass into useful bio-energy (i.e., fuel gas and bio-oil) and bio-char. Among them, biomass pyrolysis or gasification is recognized as one of the most promising technologies for producing sustainable fuels that can be used for power generation systems or syngas applications. For example, biomass pyrolysis at relative higher temperatures can produce bio-char, bio-oil and syngas for boiler and power generation. Moreover, the proportion of the derived products mainly depends on the pyrolysis temperature and other conditions. Compared with the partial oxidative gasification process, the inert pyrolysis of biomass has low process efficiency, but can produce fuel gas with a high heating value.3 Gasification of biomass has several environmental merits over fossil fuels, namely lower emission of CO2 and other flue gases (e.g., H2S, SO2, and NOx).4–8 Biomass gasification involves incomplete combustion to yield a gaseous product referred to as syngas that mainly consists of H2, CO, CH4, CO2, and N2 (if air or N2 is used as the carrier gas). Biomass pyrolysis or gasification has considerable advantages compared with direct combustion. The main reasons are that it can convert low-value feedstocks to high quality combustible syngas, which can be not directly burned for electricity generation but turned into liquid transportation fuels.9
Biomass gasification is an intricate process involving drying the feedstock followed by pyrolysis, partial combustion of intermediates, and finally gasification of the resulting products. It is usually performed in the presence of a gasifying medium, e.g., air, O2, steam (H2O) or CO2 in a gasifier. The gasification process is an alternative thermochemical method that can convert biomass into gaseous products (e.g., syngas) at relatively high temperatures. The gasification routes are shown in Fig. 1A. And the related reactions including heterogeneous and homogeneous ones in biomass gasification are depicted in Fig. 1B.10 The calorific value of the product gas is dependent on the gasifying agent.11 The product gas from air gasification gives a heating value of 4–7 MJ Nm−3 whereas when gasifying utilizing pure O2, the heating value can be as much as 12–28 MJ Nm−3.12 Biomass gasification can reduce the mass ratio of carbon-to-hydrogen (C/H) resulting in an increased calorific content of the product on account of the enhanced H2 fraction.13 The gasifying medium also plays a vital role of converting solid char and heavy hydrocarbons to low-molecular-weight gases such CO and H2. The quality and properties of the product are generally dependent on the feedstock materials, gasifying agents, feedstock dimensions, temperature and pressure inside the reactor, design of the reactor and the presence of catalysts and sorbents.14
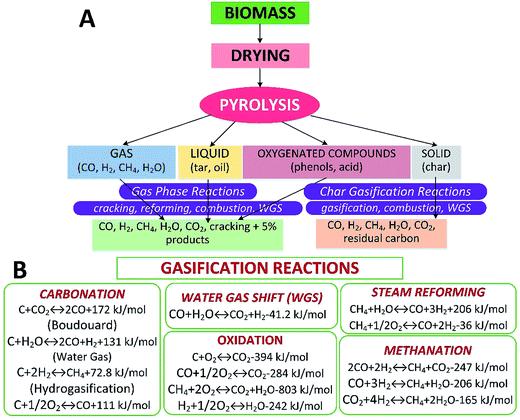 |
| | Fig. 1 Biomass gasification routes (A) and reactions (B).10 | |
Condensable organic compounds referred to as “tar” are inevitably generated with the product gas during biomass pyrolysis or gasification. Tar is regarded as one of the main bottlenecks, which limits the commercialization of biomass pyrolysis and gasification technology. Tar can condense and polymerize to form more complex structures (e.g., coke). Tar represents a complex mixture of more than one hundred compounds, including benzene, phenols, N-heterocyclic compounds, and polycyclic aromatic hydrocarbons (PAHs),15,16 which can be condensed with small particle impurities to form complex structures in downstream equipment, resulting in mechanical breakdown or catalyst deactivation.17 Various aromatic and N-heterocyclic compounds in tar are toxic and act as environmental hazards. In general, tar can be divided into primary, secondary and tertiary tars.18,19 The tar yield of biomass gasification varies from 0.5 to 100 g m−3, mainly depending on the gasifiers, operating conditions, and biomass types.20 Tar is formed in the decomposition of biomass components (namely cellulose, hemicelluloses, and lignin). The main components of primary tar include oxygenated hydrocarbons, and with increasing temperature, the oxygenated hydrocarbons are initially converted to light alkanes, olefins, and aromatics, and then transformed to higher hydrocarbons and larger PAHs with a further increase in temperature (Fig. 2).21
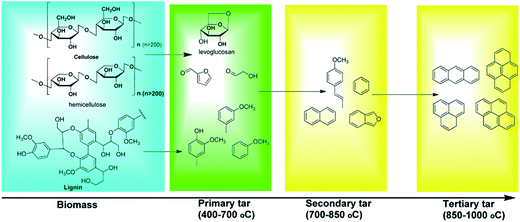 |
| | Fig. 2 Formation of tar compounds at different reaction temperatures.21 | |
Tar removal technologies could be divided into two approaches: gas downstream cleaning after the gasifier (secondary methods) and treatment inside the gasifier (primary methods) as shown in Fig. 3. The primary methods include measures taken during the gasification process to prevent or convert tar formed in the gasifier. The secondary methods are measures to improve the hot product gas exiting from the gasifier. An ideal primary method can reduce the requirement for a secondary treatment.2 The primary methods include the selection of operating conditions, bed additives or catalysts in the gasifier, and the gasifier design. The operating conditions play a crucial role in biomass gasification with many ways, such as the carbon conversion, the product gas composition, and the tar formation and reduction. The most significant influencing parameters are the temperature, the gas concentration, and the residence time. As for a given gasifier design and biomass type, these variables are the result of setting the following variables: the air ratio (i.e., oxygen feed in relation to the stoichiometric amount), the composition of the gasifying medium (flow rates of O2/steam in relation to the flow rate of biomass), and the addition of catalysts. The addition of steam can enhance the tar reforming and char gasification, improving the gas quality and reducing the tar content. However, these reactions consume heat, which must be added to the reactor, for example, by addition of O2. If O2 is added through air, the gas is diluted with N2 and the heating value of the product gas is reduced. If pure O2 is used, a better gas quality is achieved, but O2 affects the economic feasibility of the gasification process. Thus, the composition of the gasification agent must be assessed by appreciating the technical benefits and the economical drawbacks. In-bed catalysts used in fluidized-bed gasifiers have attracted much concern. The addition of various catalysts including carbonate rocks (e.g., dolomite), olivine, and metal-based catalysts can also improve significantly the gas quality by reducing the tar content. However, the loss of catalysts by entrainment, the inhibition caused by carbon and sulfur, or the contamination of ashes significantly limits these options. Again, an economic evaluation of the process determines which technique is feasible in practical use. The measure that yields the maximum reduction of tar in the bed is not necessarily the best: primary measures can be considered as a primary action in conjunction with secondary measures. The overall process should be optimized in terms of technical reliability and economic feasibility.
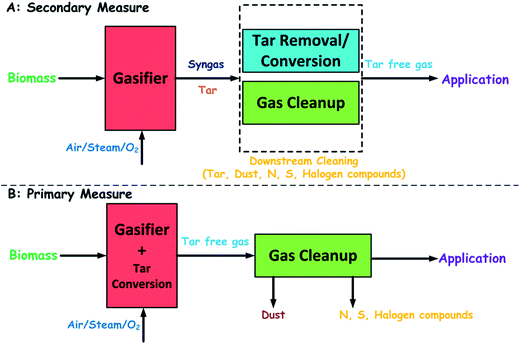 |
| | Fig. 3 (A) Primary and (B) secondary measures for gas cleaning including tar removal. | |
The secondary methods are conventionally used to treat the hot product gas of the gasifier, including chemical or physical treatments. The secondary chemical methods are composed of tar cracking/reforming in the downstream of the gasifier, either thermally or catalytically. The secondary physical methods include the use of cyclones, filters of various types (baffle, ceramic, and fabric), rotating particle separators, electrostatic filters, and scrubbers. Tar removal by condensation is, in principle, the least complicated way to remove tars. Various cooling methods have been used in biomass gasification systems, including scrubbers, venturies, humidified packed beds, etc. In direct water-cooled tar-removal systems, some of the dust, HCl, sulfur oxides, and alkaline metals can also be removed by water (e.g., venturi scrubbers). A significant drawback is the downstream wastewater contaminated by tar, which needs further treatment.20 Recently, many research studies showed that biochar can be used as a carbon catalyst or support with fair performance in tar reduction. However, a comprehensive review is required to conclude the tar catalytic reforming with biochar catalysts. The scope of this review aims to summarize the catalytic reforming of tar with various biochar catalysts derived from biomass pyrolysis or gasification. Consequently, the environmentally friendly and economical disposal of waste biochar catalysts will be discussed.
2. Catalytic reforming of tar
Several methods including physical methods (e.g., absorption and adsorption)22–25 and chemical methods (e.g., thermal/plasma-assisted cracking26,27 and catalytic reforming28,29) have be widely used for tar elimination in the primary or secondary treatment. Among them, catalytic reforming has been considered to be one of the most promising in large-scale applications because of its fast reaction rate and reliability29 and its ability to transform tar into high-value added gases such as CO and H2 in the presence or absence of steam. Various types of catalysts such as calcined rocks,30,31 zeolites,32,33 iron ores,34,35 alkali metals,36 nickel-based catalysts,37,38 and noble metal-based catalysts39 have been developed for their usefulness in tar reforming in biomass gasification (Table 1). With regards to the catalytic reactivity and economic reasons, nickel-based catalysts are considered to be one of the most promising catalysts for tar removal and gas upgrading.40–48 Nickel-based catalysts are commonly supported by natural materials (e.g., dolomite and olivine) or metal oxides (e.g., Al2O3 and MgO).1,2,29 However, these catalysts and supports are relative expensive and unsustainable. As an alternative, the by-product of char can be used as a sustainable catalyst or support with fair performance in tar removal.17 Compared with other catalysts, biochar catalysts with low cost can also be directly gasified to recover the energy of char without the requirement of frequent regeneration after deactivation.49
Table 1 Advantages and disadvantages of various catalysts for tar removal
| Catalyst type |
Advantage |
Disadvantage |
| Calcined rocks |
1. Inexpensive and abundant; 2. attain high tar conversion (>95% conversion with dolomite); 3. often used as guard beds for expensive catalysts most popular for tar reduction |
Fragile materials and quickly eroded from fluidized-bed gasifiers |
| Olivine |
1. Inexpensive; 2. high attrition resistance |
Low catalytic activity compared to dolomite |
| Clay minerals |
1. Inexpensive and abundant; 2. fewer disposal problems |
1. Lower catalytic activity compared to dolomite; 2. most natural clays do not support the high temperatures (800–850 °C) needed for tar reduction (loose porous structure) |
| Iron ores |
Inexpensive and abundant |
1. Rapidly deactivated in the absence of H2; 2. lower catalytic activity compared to dolomite |
| Fluid cracking catalysts (FCCs) |
1. Relatively inexpensive but not cheaper than the above; 2. more known about them from experience with FCC units |
1. Rapid deactivation by coke; 2. lower catalytic activity compared to dolomite |
| Alkali/alkaline-earth metals (AAEMs) |
1. Natural production in biochar; 2. reduce ash-handling issues; 3. relatively high tar conversion |
1. Particle agglomeration at high temperatures; 2. lower catalytic activity compared to dolomite |
| Activated alumina (Al2O3) |
High tar conversion comparable to that of dolomite |
Rapid deactivation by coke |
| Transition-metals |
1. Attain complete tar reduction at about 900 °C; 2. high tar conversion compared with other catalysts |
1. Rapid deactivation due to sulfur and high tar content in the feed; 2. relatively expensive; 3. more easly regenerated. |
| Char |
1. Inexpensive and abundant; 2. natural production after the process (sustainable); 3. high tar conversion compared to dolomite; 4. neutral or weak base properties |
1. Consumption because of gasification reactions; 2. unfixed properties depending on biomass types and process conditions |
Tar reforming by biochar catalysts can be performed by in situ catalysis in a gasifier or ex situ catalysis in a reformer (as shown in Fig. 4). In general, catalysts placed inside a gasifier cannot improve the conversion efficiency of biomass, but adjust the distribution of volatilized products. Moreover, tar catalytic cracking/reforming has been proved to be effective for thermochemical conversion of biomass to improve the gas yield/quality, reduce the tar content, and enhance the conversion rate.29 Biomass is decomposed in the first stage, and then the derived pyro-gases, volatiles, and tar are reformed in the second stage. The addition of catalysts along with/without steam in the second stage has a positive effect on biomass to gas conversion and tar reduction.
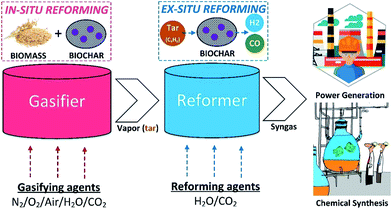 |
| | Fig. 4 Schematic illustration of in situ and ex situ reforming of tar with biochar-based catalysts during biomass pyrolysis or gasification for syngas production. | |
The mechanisms of biomass gasification and tar cracking/reforming can be expressed by means of partial combustion reactions, heterogeneous reactions and homogeneous reactions shown as below reactions (R1–R14). Among them, catalytic reforming reactions cleave the C–C bonds of the carbohydrate backbone to yield a combination of CO and H2 (R3–R5).50 If H2O is present, CO2 can be generated via the water–gas-shift reaction to produce more H2 (R14). Aqueous-phase reforming by bimetallic catalysts can produce H2 and CO2 starting with sugars and sugar alcohols.51 The selectivity towards H2 can be controlled using catalysts with different metal composites (e.g., Pt) and metal alloys (e.g., Raney Ni–Sn).52 Steam reforming can produce CO and H2 from tar. It is an endothermic process, and an external source of heat is required. Steam reforming ideally should be conducted at a high temperature, low pressure, and high steam to hydrocarbon ratio in order to achieve maximum conversions. According to Le Chatelier's principle, higher temperature is favor of the reactants in exothermic reactions and the products in endothermic reactions.53 Increasing temperature can promote the endothermic reactions (R4) and (R5) of hydrocarbons occurred in the steam reforming process, which resulted in remarkable tar removal efficiency and a slight increase of CO and H2 concentrations. The reactions (R2)–(R7) were based on the thermal catalytic cracking and reforming mechanisms, converting tar and light hydrocarbons into H2 and CO.54,55 In addition to the above mechanisms, the reactions (R8)–(R13) may also take place in the presence of char as a catalyst support.
| | | Gasification reaction: CxHyOz → H2 + CO + CO2 + CH4 + C2 + tar + char | (R1) |
| | | Thermal cracking: Tar → C + CnHm + gases | (R2) |
| | | Dry reforming: CnHm + nCO2 → 2nCO + (m/2)H2 + Q | (R3) |
| | | Steam reforming: CnHm + nH2O → nCO + (m/2)H2 + Q | (R4) |
| | | CnHm + 2nH2O → nCO2 + (m/2 + 2n)H2 + Q | (R5) |
| | | Methanation: CO2 + 4H2 ↔ CH4 + 2H2O | (R7) |
| | | Oxidation reaction: C + 1/2O2 → CO, ΔH298 K = −110.7 kJ mol−1 | (R9) |
| | | C + O2 → CO2, ΔH298 K = −405.8 kJ mol−1 | (R10) |
| | | Boudouard reaction: C + CO2 → 2CO, ΔH298 K = +172.1 kJ mol−1 | (R11) |
| | | Water gas reaction: C + H2O → CO + H2, ΔH298 K = +131.3 kJ mol−1 | (R12) |
| | | C + 2H2O → CO2 + 2H2, ΔH298 K = +89.7 kJ mol−1 | (R13) |
| | | Water gas shift (WGS): CO + H2O → CO2 + H2, ΔH298 K = −41.2 kJ mol−1 | (R14) |
3. Tar reforming with biochar catalysts
Biochar is a low-cost carbon-rich material derived from biomass via pyrolysis with limited oxygen or hydrothermal carbonization (HTC).56 The possible formation pathways of biochars including hydrochar and pyrochar vary significantly according to the reaction medium (as illustrated in Fig. 5 and 6, respectively).57,58 The advantageous properties mainly including a relatively large surface area, high pore volume, long-term stability, and enriched surface functional groups have endowed biochar with a broad spectrum of potential applications.59 However, if used for catalysts or catalyst supports, biochar directly obtained from the pyrolysis of biomass shows a relatively low specific surface area (compared with activated carbons), poor porosity, and limited surface functional groups.60 Fortunately, these properties of biochar can be easily tuned by appropriate activation or functionalization processes.57,61,62
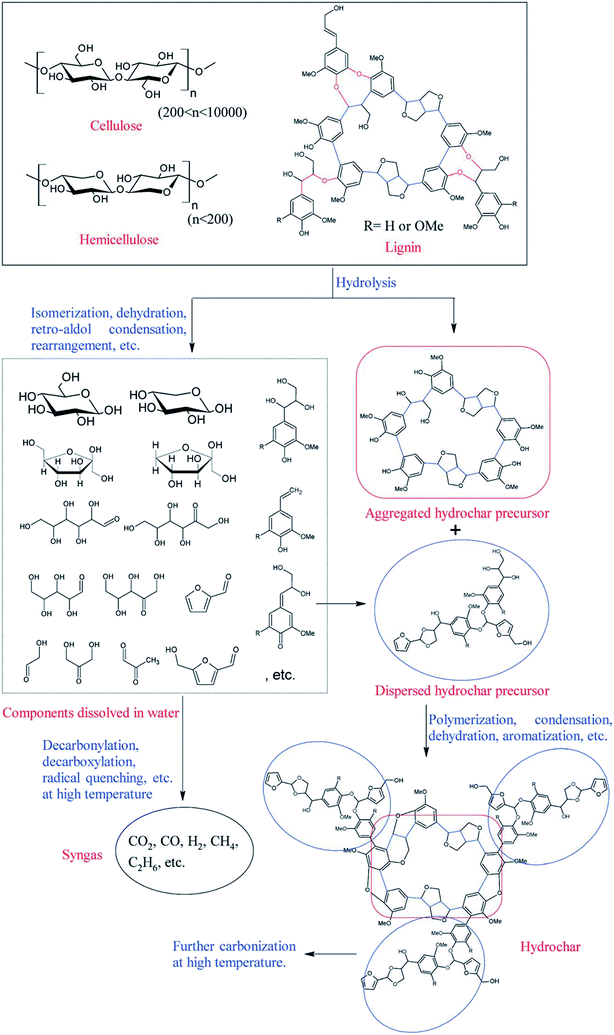 |
| | Fig. 5 Possible formation pathways of hydrochar.58 | |
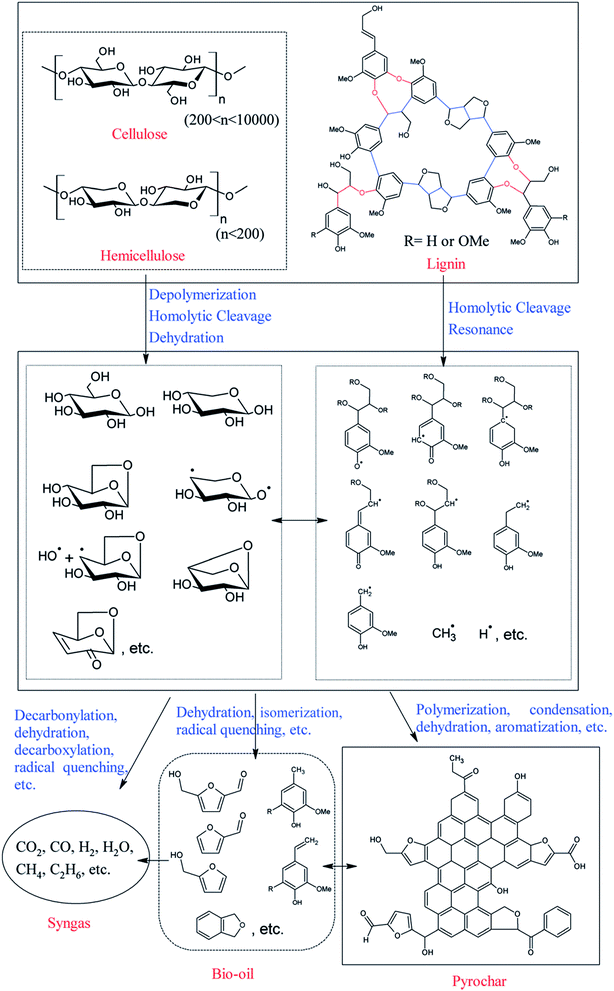 |
| | Fig. 6 Possible formation pathways of pyrochar.58 | |
In addition, the attractiveness of biochar as a catalyst for biomass upgrading originates from low cost and natural production inside the gasifier.63–65 However, it can be consumed by steam or CO2 in the product gas. The need for a continuous external char supply or withdrawal depends on the balance of char consumption and production in the entire gasification system. Interestingly, biochar also shows a good catalytic activity for tar conversion. Fuentes-Cano et al.66 studied the catalytic decomposition of tar (e.g., toluene and naphthalene) over three different char materials (coconut char, coal char, and sewage sludge char). The main mechanisms of tar conversion over carbonaceous materials mainly include deposition, dehydrogenation (soot formed on the char surface), and soot gasification.67–70 The alkali and alkaline earth metallic species (AAEMs) in biochar can also influence the gasification rate of soot.68–70 The rate of homogeneous tar reforming below 900–1000 °C (ref. 71) is smaller than that of heterogeneous tar conversion on the carbon surface.72 Significantly, reforming of aromatic molecules (e.g., benzene and naphthalene) over char cannot form other aromatic compounds.67 In contrast, alkyl- and heteroatomic compounds produce lighter compounds via dealkylation and decarboxylation.71 As illustrated in Fig. 7, the tar compounds initially come into contact with fresh char along with the active sites distributed on the surface. The tar is then adsorbed on the char matrix and undergoes polymerization reactions, producing hydrogen and soot, the latter staying over the char surface as solid deposits. This soot blocks the active sites, hindering the interaction of the active sites with the gaseous tar. If the carbon deposition rate is much higher than the carbon consumption rate, soot accumulation over the surface will take place, decreasing the number of active sites available for reaction with the tar molecules and then the char reactivity. The active sites can be attributed to the presence of AAEMs in the fresh char, since these species are known to be active during steam gasification of soot generated after tar deposition on the char surfaces.66,70
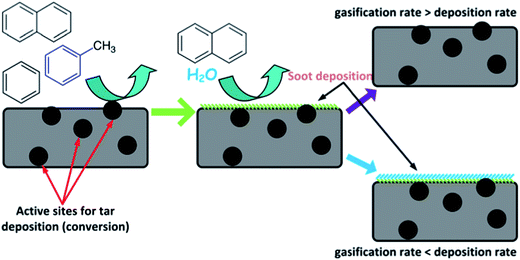 |
| | Fig. 7 Mechanism of tar compound conversion over a carbonaceous surface.66 | |
Ex situ reforming
The use of char as a material (e.g., as an adsorbent, catalyst or support) to reduce tar has been widely reported.17 As the carbon-rich product of pyrolysis, char has a large microscopic surface area due to variable-sized pores on its surface. Since it is produced by pyrolysis alongside tar, utilizing char could be an ideal way to remove tar without consumption of expensive catalysts. The main challenge for char catalysts used for tar reforming is attributed to several unstable factors such as char characteristics, reforming conditions (e.g., temperature), loaded metal types (e.g., Ni, Fe, and K), and gasification/reforming agents (e.g., CO2 and H2O).17 Tar elimination with char catalysts can be performed by in situ or ex situ reforming. The formation and thermal decomposition of tar during the gasification process is an extremely complex multi-step reaction,2 which generally involves both homogeneous conversion and heterogeneous reforming. It was proved that biochar can be effective for cracking phenol and naphthalene (two model compounds of tar) at 700–900 °C.72 Besides, Burhenne et al.73 compared the room-temperature adsorption and high-temperature (850–1050 °C) catalytic conversion of benzene (a model compound of primary tar) over different chars. The study proved that the micro- and mesoporous structure of char was especially efficient for the absorbance of benzene, and that char activated with CO2 is the most effective in benzene reduction. Furthermore, Nestler et al.74 studied the impact of char surface characteristics on the catalytic decomposition of naphthalene (a model compound of secondary tar). It was shown that wood char had no significant effect on the naphthalene decomposition at 850–1050 °C. And the naphthalene conversion was mainly influenced by thermal cracking. However, physically activated wood char (e.g., CO2 activation) can significantly increase the naphthalene conversion. While many studies have investigated the removal of model tar compounds or the performance of char catalysts,75 several studies have reported the cracking behavior of primary tar directly within the fixed bed of hot char particles.76–82 Gilbert et al.76 studied wood char with large particles (10–15 mm) for the removal of primary tar up to 65% at 800 °C. In contrast, tar removal efficiency can reach as high as 97% at 800 °C by using spruce char (particle size: ca. 1 mm)77 and rice straw char (particle size: ca. 0.15–0.42 mm).78 In the above studies, the reaction conditions for tar removal with char, mainly including the flow rate and type of purge gas (e.g., N2, O2, steam, or CO2), type and size of char and the reactor, and mass ratio of char/biomass, were quite different. Therefore, more fundamental studies are required to comprehensively understand the role of char in the reduction of primary tar under different reaction conditions.
Park et al.82 studied the impact of biochar properties and reaction conditions on the conversion of primary tar from biomass pyrolysis. As illustrated in Fig. 8A, the primary tar vapor produced from reactor 1 in biomass pyrolysis at 500 °C was passed through a fixed bed of hot biochar particles in reactor 2 for tar cracking. The used biochars had different characteristics in terms of internal pore distribution and microscopic surface area (Fig. 8B). The wood char had large pores of 10–100 μm in diameter originating from its vascular structure with a specific surface area of 93 m2 g−1. The paddy straw char had particles comprising thin plates without as many pores as the wood char with well-developed macropores of 10 nm to 1 μm in diameter, and its specific surface area was 46 m2 g−1. Besides, the palm kernel shell (PKS) char had a dense matrix with many micropores (<50 nm), which had a relatively high specific surface area of 191 m2 g−1. Ideally, the total carbon yield for the pyrolysis and thermal tar cracking should be 100%, while that for tar cracking by char can be influenced by the formation of tar deposits or char gasification. After pyrolysis, the primary tar at 500 °C has the carbon yields of 19.2% in the aqueous fraction and 12.9% in the heavy phase. By thermal cracking of tar at higher temperatures cracking at higher temperatures, the carbon yield of tar is reduced from a total of 32.1% to 19.9% at 800 °C. In the presence of hot wood char particles, the carbon yield of the aqueous fraction is greatly reduced to 2.8% at 800 °C. Also, the carbon yield of the heavy fraction is significantly reduced to 8.7%, suggesting that hot biochar is effective in enhancing tar decomposition.82 As reported by Zhang et al.,80 alkali metals in straw char could play an important role in the catalytic cracking of tar and that the catalytic role of biochar promoted the generation of alkyl mono-aromatics and suppressed the generation of polycyclic aromatic hydrocarbons (PAHs) from primary tar.
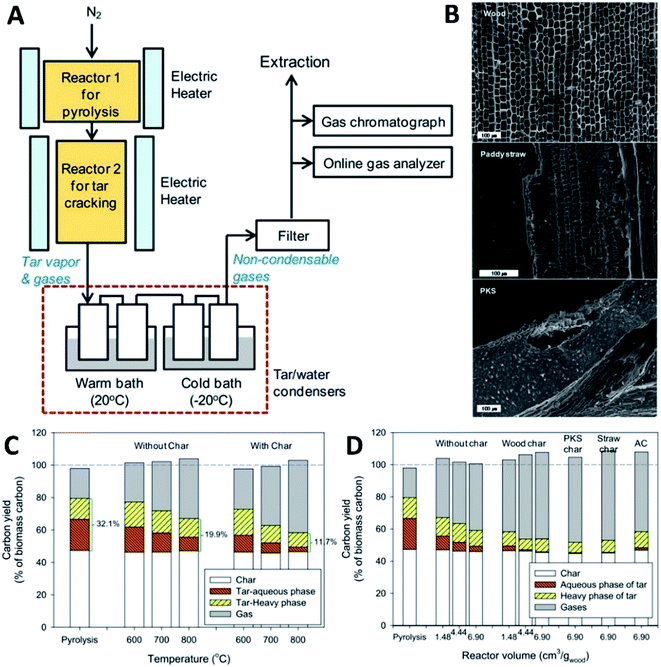 |
| | Fig. 8 (A) Schematic of pyrolysis and tar-cracking reactors, (B) SEM images of biochars: wood char, paddy straw char and PKS char, (C) carbon yield of products from tar cracking with/without char at different temperatures (wood char, VTR = 1.48 cm3 g−1 wood), and (D) carbon yield of products from tar cracking with/without char for different reactor volumes at Tc = 800 °C.82 | |
In real biomass gasification processes, H2O and CO2 are present during the tar cracking process and simultaneously reactivate the active sites of the char. As carbon degradation thereby reduces coke deposition, the high activity of the catalyst micropores can be obtained.74 Steam (H2O) is commonly used as the gasifying agent.83,84 Furthermore, advanced gasification technologies85 can produce CO2 in a form that is suitable for direct sequestration/storage.86 Therefore, CO2 gasification of biomass can offer near-zero emissions with high thermal efficiency.
Toluene (C7H8) is one of the volatile benzenes, which has been regarded as a model compound of tar.87 The main reactions involved in toluene cracking are shown as follows (R15–R24). Huang et al.88–90 studied the cracking of toluene with real waste-derived biochar catalysts. The results show that the cracking efficiency can be ordered as NiO/ϒ–Al2O3 > calcined dolomite > dry sewage sludge (DSS) char > ash.88 The inherent AAEM species in biochar play an important role in catalytic steam reforming of tar. Acid-treatment of biochar can significantly reduce the contents of AAEM species and the activity of biochar. The inherent AAEM species have a significant effect on the WGS reaction and an insignificant effect on the steam reforming methane reaction.91 Although the waste-derived biochar shows a considerable catalytic performance in the reforming of pyrolysis volatiles (e.g., oil and tar),92 its catalytic activity is required to be enhanced. Biochar with H2O/CO2 activation and nickel loading can enhance the catalytic activity for tar cracking.90 As a catalyst or catalyst support, raw biochar should be initially activated to obtain a higher and more stable catalytic activity for tar conversion. In addition, biochar loaded with relatively cheap metal species such as Ni,93–95 Fe,96,97 and AAEMs98,99 can significantly enhance the catalytic activity for tar cracking. Among them, Ni-based catalysts have been used widely for tar conversion because of their high tar cracking activity. Nevertheless, the main limitation of nickel catalysts is their rapid deactivation, caused by carbon formation on the catalyst surface.100–102 Moreover, other metal catalysts like Co, Fe, Zn, and Cu have been employed in steam reforming of tar and show higher catalytic activity than Ni catalysts in some cases.29 Although other metal catalysts exhibit a good performance in steam reforming of tar, they are still deactivated easily by sulfur or high heavy tar content.29 Kaewpanha et al.103 studied the steam reforming of tar over a biomass char supported molybdenum carbide (Mo2C/BC) catalyst in a fixed-bed reactor. The Mo2C/BC showed a good catalytic activity for steam reforming of tar. And the deactivated biochar catalyst can be regenerated.
| | | Thermal cracking: C7H8 → coke + 2H2 + CH4 | (R15) |
| | | Catalytic cracking: C7H8 → coke + H2 + CH4 + CO + CO2 | (R16) |
| | | Hydrocracking: C7H8 + H2 → coke + 2H2 + CH4 | (R17) |
| | | Dry reforming: C7H8 + 7CO2 → 14CO + 4H2, −1157 kJ mol−1 | (R18) |
| | | Steam reforming: C7H8 + 7H2O → 7CO + 11H2, −869 kJ mol−1 | (R19) |
| | | C7H8 + 14H2O → 7CO2 + 18H2, −581 kJ mol−1 | (R20) |
| | | Methanation: CO + 3H2 ↔ CH4 + H2O, +206 kJ mol−1 | (R21) |
| | | Boudouard: C + CO2 → 2CO, −172.1 kJ mol−1 | (R22) |
| | | Water gas: C + H2O → CO + H2, −131 kJ mol−1 | (R23) |
| | | Water gas shift (WGS): CO + H2O → CO2 + H2, +41.2 kJ mol−1 | (R24) |
The synthesis of low cost, highly active, robust and coke-resistant catalysts to facilitate tar removal and improve the overall conversion efficiency in biomass pyrolysis or gasification is still one of the most urgent research topics. Various innovative synthetic methodologies of highly functionalized metal nanoparticles (NPs) have attracted considerable attention.104 Gai et al.105,106 synthesized hydrochar-supported iron NPs by a one-pot hydrothermal carbonization (HTC) process (as illustrated in Fig. 9A). Compared with the impregnation approach, a nanocatalyst with uniformly dispersed Fe NPs immobilized on hydrochar was produced by the one-pot synthesis. And the particle size of the Fe NPs and the surface area of the nanocatalysts produced via the one-pot synthesis can be manipulated (Fig. 9B). The nanocatalysts prepared by the one-pot synthesis exhibited higher catalytic activity in thermal decomposition of phenol (biomass tar model compound) at mild temperatures and resistance to coke deposition.105 In the HTC process, nanosized channels or pits can be formed on the hydrophilic shell and coating of the metal NPs into the hydrophobic core of the hydrochar under a relatively high pressure. And the uniformly distributed pores on the hydrochar surface can promote the penetration of reactive species and Ni2+ cations. The nickel NPs will be preferentially dispersed in situ in the hydrophobic core of the hydrochar to form inner-sphere surface complexes, which contribute to enhanced interaction between the nickel NPs and the supports during the preparation process and restrain the rapid growth of the nickel NPs during the gasification process. Furthermore, the grain size of the in situ-generated NiO NPs decorating the hydrochar nanospheres can be tailored by varying the concentration of the precursory Ni2+ during the one-pot HTC process. These nickel-based nanomaterials can offer promising potential for H2-rich syngas production along with tar reduction in the biomass gasification process.106
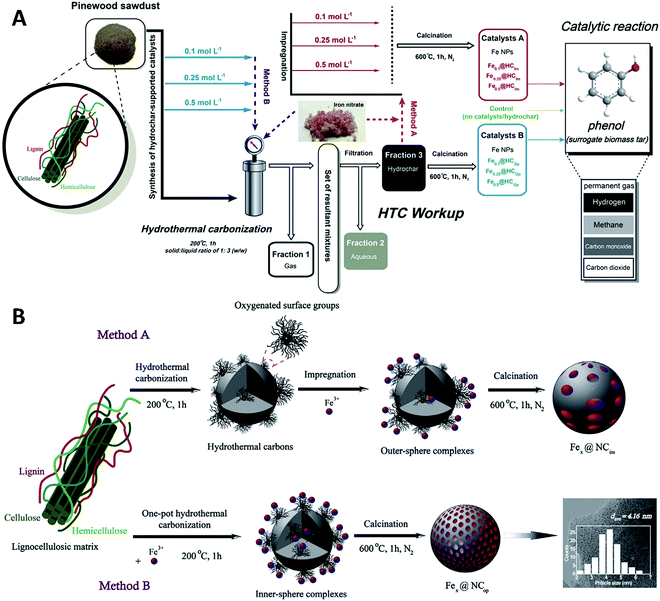 |
| | Fig. 9 (A) Preparation of iron-based nanomaterials by the impregnation approach (method A) and one-pot synthesis (method B); (B) schematic of the synthesis of iron NPs supported on hydrochar from a lignocellulosic matrix.105 | |
In situ reforming
The development and implementation of advanced gasification and gas cleaning up technologies have been proposed and remain the subject of intense, highly interdisciplinary research within the scientific community working on catalysis, bioengineering, chemical reaction engineering, and process engineering.107–112 Currently, three main approaches of process intensification in the syngas production process from biomass are considered and consist of (1) incorporation of cleanup multifunctional systems into existing gasification reactors (e.g., catalytic hot gas filters),113,114 (2) implementation of specific highly reactive gasification media such as supercritical water115–117 or molten metals,118–120 and (3) development of efficient integrated catalytic gasification reactors and concepts (e.g., in situ tar reforming and chemical looping gasification).107,110,121–123 The former two approaches have limited potential for the reduction of a plant size/production capacity ratio, but the third one turns out to be more promising for dramatically decreasing the economically viable size compared to existing processes through the development of new high-efficiency, small-sized catalytic reactors. These intensification approaches consist of different strategies of catalytic integration that target both the reduction of biomass residence time in gasifiers and simplification of downstream syngas treatment before its subsequent catalytic conversion.
Richardson et al.124 proposed a novel concept of integrated catalytic biomass pyrolysis or gasification, including different key reaction steps (as illustrated in Fig. 10), consisting of (step 1) metal precursor (e.g., Ni2+) insertion into biomass, (step 2) the catalytic pyrolysis of biomass, (step 3) catalytically active NP (e.g., Ni0) in situ generation and high dispersion in the solid fuel, (step 4) the catalytic conversion of nascent tars by the formed metal NPs (e.g., Ni0), (step 5) the catalytic gasification of the biochar composite (the biochar–Ni0 catalyst showing a high activity towards tar conversion and char gasification),125–128 and (step 6) the recycling of the metal catalyst in the ash (e.g., NiO/SiO2). It is noteworthy that the integrated catalytic biomass pyrolysis can achieve the in situ synthesis of metal NPs in the char matrix, the in situ reduction of tar and the in situ upgrading of syngas.129 Each of the steps requires a fundamental understanding to develop a high-efficiency biomass gasification process at the nanoscale. In addition, it is critical to develop breakthrough conversion technologies with the design of future intensified gasification processes. In this integrated concept, two kinds of in situ tar reforming routes are involved: (1) catalytic decomposition of biomass to produce less tar (namely catalytic pyrolysis) and (2) catalytic conversion of nascent tars derived from biomass co-pyrolysis with biochar catalysts (namely pyrolysis-catalytic cracking). For example, Liu et al.130 recently investigated a novel pyrolysis process (called autocatalytic pyrolysis) with wastewater biosolid-derived biochar (WB-biochar) as a catalyst to reduce bio-oil and increase the pyrolysis gas (i.e. py-gas) yield for easier energy recovery. The WB-biochar increased the py-gas yield nearly 2-fold. The metals in wastewater biosolids could play an important role in upgrading pyrolysis products. Especially, the Ca and Fe in the WB-biochar reduced the bio-oil yield and increased the py-gas yield.
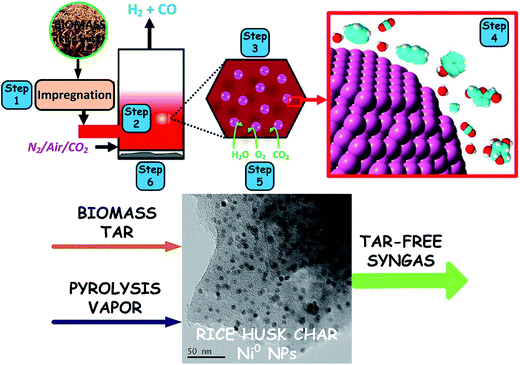 |
| | Fig. 10 Schematic of integrated catalytic biomass pyrolysis/gasification2,107 | |
As for the in situ reduction of tar, co-pyrolysis with biochar catalysts has been studied widely by catalytic cracking/reforming of nascent tar derived from biomass pyrolysis. Using waste materials (like biochar) more conveniently to produce catalysts in addition to the target product makes the system more cost effective and environmentally friendly.131 Compared with the catalytic pyrolysis of biomass preloaded with metals to produce tar-free syngas, co-pyrolysis with biochar or supported catalysts is more efficient and economical. Wang et al.132 investigated Ni-based catalysts prepared by mechanically mixing NiO and char particles at various ratios. The catalyst preparation is inexpensive and convenient without impregnation or calcination steps. Yu et al.133,134 directly used biochar or biochar–Fe catalysts for in situ reforming of volatiles derived from sewage sludge pyrolysis. In addition, Song et al.135–137 studied the interactions between volatiles and char for in situ destruction of nascent tar. The oxygen-containing functional groups in biochar play a significant role in in situ tar reforming, and the disordered structure determined the catalytic activity of AAEMs in biomass gasification. The chemical structure of the char surface is important for tar destruction during the volatile–char interactions. However, it can also be consumed by steam or CO2 in the producer gas. The requirement for a continuous external char supply and/or withdrawal mainly depends on the balance of char production and consumption in the gasification system.2
In summary, the high catalytic performance of biochar for tar reforming is attributed to its specific surface properties (relatively large surface area), amorphous carbon structure with active surface functional groups, and the properties of metal species (e.g., concentration, physicochemical state and dispersion) such as AAEMs in the biochar matrix. However, one of the main concerns with this process in homogeneous and heterogeneous reforming of tar is catalyst deactivation, which can be caused by coke formation, thus blocking the active sites in biochar and reducing the surface area of biochar. Besides, the catalyst loss can occur as biochar is consumed by the steam or dry reforming reactions. AAEMs in biomass can play an important role as “cross points” during tar formation. The chemical bonds between AAEMs and the carbon matrix are repeatedly breaking and reforming. This process promotes the generation of gaseous products from the fatty acid tar and a few of small aromatic compounds. Meanwhile, larger aromatic ring compounds can be formed with the biochar structure. The presence of AAEMs can inhibit the release of biomass tar.
Feng et al.138–142 investigated the effects of AAEMs and gasifying/reforming agents (i.e. H2O and CO2) on tar reforming with biochar. The in situ volatile (tar and free radicals) H2O reforming over nascent biochar could be conducted in three ways: occupying reactive sites on biochar, changing biochar structures and/or changing the total concentration of AAEMs. The mechanisms of in situ tar H2O reforming by K and Ca species were different: tar cracking into light tar or small-molecule gas could be catalyzed by K, while the combination of tar with biochar was promoted by Ca. The volatilizations of K and Ca in the presence of volatiles were in accordance with their valences (i.e. monovalent K+ and divalent Ca2+) and their boiling points. The subsequent transformation from the small aromatic rings to larger ones could be attribute to the volatile–biochar interaction. During the in situ tar H2O reforming over biochar, K and Ca can act as the active sites on the biochar surface to promote the increase of active intermediates (i.e., C–O bonds and C–O–K/Ca).139Fig. 11 shows the reforming of tar model compounds with biochar under a H2O/CO2 atmosphere at 800 °C. For the H2O or CO2 reforming of phenol with biochar, the decomposition rate can remain stable at nearly 100.00% (Fig. 11B). The K-loaded char is significant for tar decomposition in H2O or CO2.
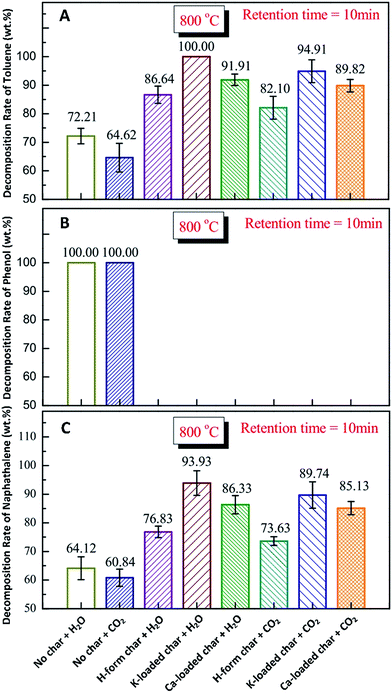 |
| | Fig. 11 Decomposition rates of model tar compounds of toluene (A), naphthalene (B) and phenol (C) with biochar under a H2O/CO2 atmosphere at 800 °C.140 | |
The reforming mechanism of tar with H-form/K-loaded/Ca-loaded biochars under H2O/CO2 at 800 °C can be also illustrated (see Fig. 12). The active oxygen atoms for oxidative decomposition of hydrocarbons and intermediate products are mainly produced by the reaction CO2 + e* = CO + O˙ + e. Active OH free radicals are formed by replacing the hydrogen atoms in the hydrocarbons with oxygen atoms. In a steam atmosphere, O and OH free radicals can be formed by ionization of H2O (H2O = ˙H + ˙OH). The fracture of OH can form new H and O free radicals. However, the process is not strong. The H/O/OH atoms in the gas phase exist in radical form. These free radicals can play a vital role in transforming AAEM species from metallic to radical forms. The CM–Ca and CM–K bonds are not stable at high temperatures in H2O or CO2 atmospheres and may be broken to generate active sites together with the release of O-containing species (e.g., COx) or aliphatic materials (e.g., CH4).143,144 Some of the AAEMs leave the biochar particles (R26 and R27). New CM–Ca and CM–K bonds can be formed through recombination (R29 and R30).145 The CM–K and CM–Ca bonds can be continuously broken and reformed. Repeated CM–AAEM bond forming and bond breaking increase the concentration of active sites in the biochar particles, providing more chances for combination and cracking of tar compounds.
| | | (CM–Ca–CM) = (–CM) + (–Ca–CM) | (R25) |
| | | (–Ca–CM) = (–CM) + Ca | (R26) |
| | | (–CM) = (–CM′) + gas | (R28) |
| | | (–CM′) + (–Ca–CM) = (CM′–Ca–CM) | (R29) |
| | | (–CM′) + K = (CM′–K) | (R30) |
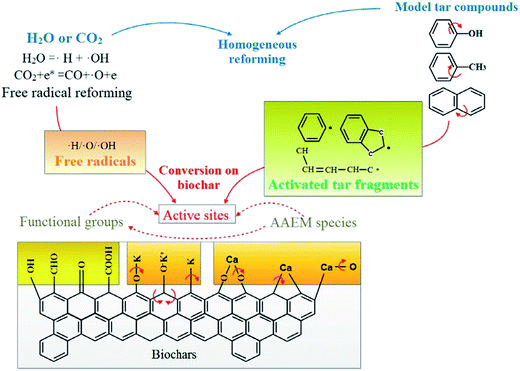 |
| | Fig. 12 Reforming mechanism of tar compounds with H-form/K-loaded/Ca-loaded biochars under a H2O/CO2 atmosphere at 800 °C.140 | |
During volatilization, AAEMs leave the gas as K+ and Ca2+ radicals. The AAEM radicals can combine with activated tar fragments in the gas formed through dealkylation and depolymerization of the tar compounds. Due to the low stability of the species formed by combination, the H/O/OH free radicals can easily exchange with the AAEMs from the activated tar fragments to form light tar. The presence of K and Ca, which lead to repeated bond forming and breaking of species formed from AAEMs and tar fragments, results in the conversion of tar compounds. After combination with free radicals, a series of bond-breaking and ring-opening reactions can result in gradual tar decomposition and the formation of light hydrocarbons and small-molecule gases. The cracking and transformation of tar is promoted by the above reactions. Tar catalytic cracking occurs on the catalyst surface, and the formation of activated tar fragments in the space can promote chemical reactions between tar fragments and H/O/OH free radicals. The reactants combined on the char active sites (AAEMs and some defects in the carbon structure) can easily participate in reforming reactions. For a biochar surface, the activated tar fragments can be combined on the active sites of the char matrix and take part in further reforming reactions. The pathways for tar reforming in H2O or CO2 by K and Ca in biochar include direct homogeneous reforming and consumption by H2O or CO2 gasification on the biochar surface. Therefore, gasification could become a promising way for the disposal of waste biochar catalysts, because the carbon source of biochar is directly converted to additional syngas.146 The catalysts (e.g., metal actives) in-site of biochar could play a significant role in the gasification process under H2O or CO2 conditions.127,147 Furthermore, the catalyst metal species in the ashes could be recycled and reused.
4. Conclusions
Thermochemical processes such as gasification can decompose carbon feedstocks in a controlled oxygen environment to transform them into gaseous, liquid, and solid char products. Gasification is generally performed in a partial-oxidation environment (e.g., air, CO2, and steam). In particular, steam gasification has attracted more attention for converting biomass into H2-rich syngas. Various catalysts (e.g., metal-based catalysts) have been successfully developed for the decomposition of model tars such as benzene, toluene, phenol and naphthalene. However, most of them are still unsuitable for real tar reforming. Moreover, for a practical biomass gasification process, in order to reform complex tars, using low-cost and disposable catalysts still attracts special attention. Biochar is the predominant by-product from biomass pyrolysis or gasification, which has high potential to be used for gas cleaning (e.g., tar removal). This paper reviewed the advances in tar reforming with biochar catalysts. Bio-char can be used as a carbonaceous catalyst or support with fair performance in tar removal. It is noteworthy that char-supported catalysts can be directly gasified to recover the energy of char without the need for frequent regeneration after deactivation. Therefore, an integrated strategy of catalytic biomass pyrolysis/gasification was proposed with the objective to promote the environmentally friendly application of biomass gasification in the future. In summary, the high catalytic performance of biochar for tar reforming is attributed to its specific surface properties, amorphous carbon structure with active surface functional groups, and the properties of metal species (e.g., concentration, physicochemical state and dispersion) such as AAEMs in the biochar matrix. Particularly, K-loaded char is significant for tar decomposition in H2O or CO2. Furthermore, biochar-based catalysts can be developed for the removal of contaminants including NH3, H2S and tar simultaneously in producer gas from real biomass gasification processes. In addition, an integrated industrial strategy could be developed through which biochar from biomass pyrolysis is activated and/or modified for wastewater treatment (e.g., industrial electroplating wastewater/sludge), followed by biochar catalyst production for tar conversion at relatively low temperatures.
Conflicts of interest
There are no conflicts to declare.
Acknowledgements
The present work is supported by the Startup Foundation for Introducing Talent at the NUIST (Grant 2243141501046). The authors also gratefully acknowledge the support by the National Natural Science Foundation of China (Grant 21407079, 91543115 and 91544220) and the Natural Science Foundation of the Higher Education Institutions of Jiangsu Province (Grant 16KJB610012).
References
- F. L. Chan and A. Tanksale, Review of recent developments in Ni-based catalysts for biomass gasification, Renewable Sustainable Energy Rev., 2014, 38, 428–438 CrossRef CAS.
- Y. Shen, J. Wang, X. Ge and M. Chen, By-products recycling for syngas cleanup in biomass pyrolysis - An overview, Renewable Sustainable Energy Rev., 2016, 59, 1246–1268 CrossRef CAS.
- J. A. Ruiz, M. C. Juárez, M. P. Morales, P. Muñoz and M. A. Mendívil, Biomass gasification for electricity generation: review of current technology barriers, Renewable Sustainable Energy Rev., 2013, 18, 174–183 CrossRef CAS.
-
P. Basu, Gasification theory and modeling of gasifiers, in Biomass Gasification Design Handbook, Academic Press, Boston, 2010, pp. 117–165 Search PubMed.
- W. Torres, S. S. Pansare Jr and J. G. Goowin, Hot gas removal of tars, ammonia, and hydrogen sulfide from biomass gasification gas, Catal. Rev., 2007, 49, 407–456 CAS.
- G. J. Stiegel and R. C. Maxwell, Gasification technologies: the path to clean, affordable energy in the 21st century, Fuel Process. Technol., 2001, 71, 79–97 CrossRef CAS.
- I. I. Ahmed, N. Nipattummakul and A. K. Gupta, Characteristics of syngas from cogasification of polyethylene and woodchips, Appl. Energy, 2011, 88, 165–174 CrossRef CAS.
- I. I. Ahmed and A. K. Gupta, Evolution of syngas from cardboard gasification, Appl. Energy, 2009, 86, 1732–1740 CrossRef CAS.
- A. V. Bridgwater, The technical and economic feasibility of biomass gasification for power generation, Fuel, 1995, 74, 631–653 CrossRef CAS.
- Y. Shen, D. Ma and X. Ge, CO2-looping in biomass pyrolysis or gasification, Sustainable Energy Fuels, 2017, 1, 1700–1729 CAS.
- V. S. Sikarwar, M. Zhao, P. Clough, J. Yao, X. Zhong, M. Z. Memon, N. Shah, E. J. Anthony and P. S. Fennell, An overview of advances in biomass gasification, Energy Environ. Sci., 2016, 9, 2939–2977 CAS.
- S. Rapagnà, N. Jand, A. Kiennemann and P. U. Foscolo, Steam-gasification of biomass in a fluidised-bed of olivine particles, Biomass Bioenergy, 2000, 19, 187–197 CrossRef.
-
C. Higman and M. v. d. Burgt, Gasification, Gulf Professional Publishing, Elsevier, USA, 2008 Search PubMed.
- P. Parthasarathy and K. S. Narayanan, Hydrogen production from steam gasification of biomass: Influence of process parameters on hydrogen yield – A review, Renewable Energy, 2014, 66, 570–579 CrossRef CAS.
- A. Dufour, P. Girods, E. Masson, S. Normand, Y. Rogaume and A. Zoulalian, Comparison of two methods of measuring wood pyrolysis tar, J. Chromatogr. A, 2007, 1164, 240–247 CrossRef CAS PubMed.
- L. Jia, Y. Le Brech, G. Mauviel, F. Qi, M. Bente-von Frowein, S. Ehlert, R. Zimmermann and A. Dufour, Online analysis of biomass pyrolysis tar by photoionization mass spectrometry, Energy Fuels, 2016, 30, 1555–1563 CrossRef CAS.
- Y. Shen, Chars as carbonaceous adsorbents/catalysts for tar elimination during biomass pyrolysis or gasification, Renewable Sustainable Energy Rev., 2015, 43, 281–295 CrossRef CAS.
- G. Guan, M. Kaewpanha, X. Hao and A. Abudula, Catalytic steam reforming of biomass tar: prospects and challenges, Renewable Sustainable Energy Rev., 2016, 58, 450–461 CrossRef CAS.
- C. Font Palma, Modelling of tar formation and evolution for biomass gasification: a review, Appl. Energy, 2013, 111, 129–141 CrossRef CAS.
-
A. Gomez-Barea and B. Leckner, Gasification of Biomass and Waste, in Handbook of Combustion, Wiley-VCH, 2010 Search PubMed.
- W. Liu, W. Li, H. Jiang and H. Yu, Fates of chemical elements in biomass during its pyrolysis, Chem. Rev., 2017, 117, 6367–6398 CrossRef CAS PubMed.
- T. Phuphuakrat, T. Namioka and K. Yoshikawa, Tar removal from biomass pyrolysis gas in two-step function of decomposition and adsorption, Appl. Energy, 2010, 87, 2203–2211 CrossRef CAS.
- A. Paethanom, S. Nakahara, M. Kobayashi, P. Prawisudha and K. Yoshikawa, Performance of tar removal by absorption and adsorption for biomass gasification, Fuel Process. Technol., 2012, 104, 144–154 CrossRef CAS.
- P. Hasler and T. Nussbaumer, Gas cleaning for IC engine applications from fixed bed biomass gasification, Biomass Bioenergy, 1999, 16, 385–395 CrossRef CAS.
- A. Paethanom, P. Bartocci, B. D. Amico, F. Testarmata, N. Moriconi, K. Slopiecka and K. Yoshikawa, A low-cost pyrogas cleaning system for power generation: Scalling up from lab to pilot, Appl. Energy, 2013, 111, 1080–1088 CrossRef CAS.
- L. Fagbemi, L. Khezami and R. Capart, Pyrolysis products from different biomasses: application to the thermal cracking of tars, Appl. Energy, 2001, 69, 293–306 CrossRef CAS.
- S. A. Nair, A. J. M. Pemen, K. Yan, E. J. M. Van Heesch, K. J. Ptasinski and A. A. H. Drinkenburg, Chemical processes in tar removal from biomass derived fuel gas by pulsed corona discharges, Plasma Chem. Plasma Process., 2003, 23, 665–680 CrossRef CAS.
- Z. Abu El-Rub, E. A. Bramer and G. Brem, Review of catalysts for tar elimination in biomass gasification processes, Ind. Eng. Chem. Res., 2004, 43, 6911–6919 CrossRef CAS.
- Y. Shen and K. Yoshikawa, Recent progresses in catalytic tar elimination during biomass gasification or pyrolysis – a review, Renewable Sustainable Energy Rev., 2013, 21, 371–392 CrossRef CAS.
- L. Devi, K. J. Ptasinski, F. J. J. G. Janssen, S. V. B. van Paasen, P. C. A. Bergman and J. H. A. Kiel, Catalytic decomposition of biomass tars: use of dolomite and untreated olivine, Renewable Energy, 2005, 30, 565–587 CrossRef CAS.
- D. A. Constantinou, J. L. G. Fierro and A. M. Efstathiou, A comparative study of the steam reforming of phenol towards H2 production over natural calcite, dolomite and olivine materials, Appl. Catal., B, 2010, 95, 255–269 CrossRef CAS.
- P. R. Buchireddy, R. M. Bricka, J. Rodriguez and W. Holmes, Biomass gasification: Catalytic removal of tars over zeolites and nickel supported zeolites, Energy Fuels, 2010, 24, 2707–2715 CrossRef CAS.
- L. Yan, X. Kong, R. Zhao, F. Li and K. Xie, Catalytic upgrading of gaseous tars over zeolite catalysts during coal pyrolysis, Fuel Process. Technol., 2015, 138, 424–439 CrossRef CAS.
- S. Hosokai, K. Matsui, N. Okinaka, K. Ohno, M. Shimizu and T. Akiyama, Kinetic study on the reduction reaction of biomass-tar-infiltrated iron ore, Energy Fuels, 2012, 26, 7274–7279 CrossRef CAS.
- Y. Mochizuki, N. Tsubouchi and T. Akiyama, Reduction behavior and crushing strength of carbon-containing iron ore sinters prepared from tar recovered from coke oven gas, Fuel Process. Technol., 2015, 138, 704–713 CrossRef CAS.
- L. D. Felice, C. Courson, P. U. Foscolo and A. Kiennemann, Iron and nickel doped alkaline-earth catalysts for biomass gasification with simultaneous tar reformation and CO2 capture, Int. J. Hydrogen Energy, 2011, 36, 5296–5310 CrossRef.
- P. Liang, X. Wang, Y. Zhang, J. Yu and X. Zhang, Effect of additives on cracking characteristics of dust-containing tar over nickel-based catalysts, Energy Fuels, 2016, 30, 5115–5121 CrossRef CAS.
- Y. Shen, P. Zhao, Q. Shao, D. Ma, F. Takahashi and K. Yoshikawa,
in situ catalytic conversion of tar using rice husk char-supported nickel-iron catalysts for biomass pyrolysis/gasification, Appl. Catal., B, 2014, 152–153, 140–151 CrossRef CAS.
- K. Tomishige, T. Miyazawa, M. Asadullah, S. Ito and K. Kunimori, Catalyst performance in reforming of tar derived from biomass over noble metal catalysts, Green Chem., 2003, 5, 399–403 RSC.
- M. Koike, C. Ishikawa, D. Li, L. Wang, Y. Nakagawa and K. Tomishige, Catalytic performance of manganese-promoted nickel catalysts for the steam reforming of tar from biomass pyrolysis to synthesis gas, Fuel, 2013, 103, 122–129 CrossRef CAS.
- M. Kong, J. Fei, S. Wang, W. Lu and X. Zheng, Influence of supports on catalytic behavior of nickel catalysts in carbon dioxide reforming of toluene as a model compound of tar from biomass gasification, Bioresour. Technol., 2011, 102, 2004–2008 CrossRef CAS PubMed.
- C. Li, D. Hirabayashi and K. Suzuki, Development of new nickel based catalyst for biomass tar steam reforming producing H2-rich syngas, Fuel Process. Technol., 2009, 90, 790–796 CrossRef CAS.
- K. Sato and K. Fujimoto, Development of new nickel based catalyst for tar reforming with superior resistance to sulfur poisoning and coking in biomass gasification, Catal. Commun., 2007, 8, 1697–1701 CrossRef CAS.
- R. Zhang, Y. Wang and R. C. Brown, Steam reforming of tar compounds over Ni/olivine catalysts doped with CeO2, Energy Convers. Manage., 2007, 48, 68–77 CrossRef CAS.
- P. H. Blanco, C. Wu, J. A. Onwudili and P. T. Williams, Characterization and evaluation of Ni/SiO2 catalysts for hydrogen production and tar reduction from catalytic steam pyrolysis-reforming of RDF, Appl. Catal., B, 2013, 134–135, 238–250 CrossRef CAS.
- X. Yang, S. Xu, H. Xu, X. Li and C. Liu, Nickel supported on modified olivine catalysts for steam reforming of biomass gasification tar, Catal. Commun., 2010, 11, 383–386 CrossRef CAS.
- T. Kimura, T. Miyazawa, J. Nishikawa, S. Kado, K. Okumura, T. Miyao, S. Naito, K. Kunimori and K. Tomishige, Development of Ni catalysts for tar removal by steam gasification of biomass, Appl. Catal., B, 2006, 3–4, 160–170 CrossRef.
- J. Ashok and S. Kawi, Nickel–iron alloy supported over iron–alumina catalysts for steam reforming of biomass tar model compound, ACS Catal., 2014, 4, 289–301 CrossRef CAS.
- Y. Shen, M. Ding, X. Ge and M. Chen, Catalytic CO2 gasification of rice husk char for syngas and silica-based nickel nanoparticles production, Ind. Eng. Chem. Res., 2015, 54, 8919–8928 CrossRef CAS.
- E. L. Kunkes, D. A. Simonetti, R. M. West, J. C. Serrano-Ruiz, C. A. Gärtner and J. A. Dumesic, Catalytic conversion of biomass to monofunctional hydrocarbons and targeted liquid-fuel classes, Science, 2008, 322, 417–421 CrossRef CAS PubMed.
- R. D. Cortright, R. R. Davda and J. A. Dumesic, Hydrogen from catalytic reforming of biomass-derived hydrocarbons in liquid water, Nature, 2002, 418, 964–967 CrossRef CAS PubMed.
- G. W. Huber, J. W. Shabaker and J. A. Dumesic, Raney Ni–Sn catalyst for H2 production from biomass-derived hydrocarbon, Science, 2003, 300, 2075–2077 CrossRef CAS PubMed.
- M. R. Mahishi and D. Y. Goswami, Thermodynamic optimization of biomass gasifier for hydrogen production, Int. J. Hydrogen Energy, 2007, 32, 3831–3840 CrossRef CAS.
- E. G. Baker, L. K. Mudge and M. D. Brown, Steam gasification of biomass with nickel secondary catalysts, Ind. Eng. Chem. Res., 1987, 26, 1335–1339 CrossRef CAS.
-
L. Mudge, E. G. Baker, M. D. Brown and W. A. Wilcox, Bench-scale Studies on Gasification of Biomass in the Presence of Catalysts, Pacific Northwest Laboratory, Richland (WA, USA), 1987 Search PubMed.
- X. Tan, Y. Liu, G. Zeng, X. Wang, X. Hu, Y. Gu and Z. Yang, Application of biochar for the removal of pollutants from aqueous solutions, Chemosphere, 2015, 125, 70–85 CrossRef CAS PubMed.
- H. S. Kambo and A. Dutta, A comparative review of biochar and hydrochar in terms of production, physico-chemical properties and applications, Renewable Sustainable Energy Rev., 2015, 45, 359–378 CrossRef CAS.
- X. Cao, S. Sun and R. Sun, Application of biochar-based catalysts in biomass upgrading: a review, RSC Adv., 2017, 7, 48793–48805 RSC.
- X. Xiong, I. K. M. Yu, L. Cao, D. C. W. Tsang, S. Zhang and Y. S. Ok, A review of biochar-based catalysts for chemical synthesis, biofuel production, and pollution control, Bioresour. Technol., 2017, 246, 254–270 CrossRef CAS PubMed.
- W. Liu, H. Jiang and H. Yu, Development of biochar-based functional materials: Toward a sustainable platform carbon material, Chem. Rev., 2015, 115, 12251–12285 CrossRef CAS PubMed.
- J. S. Cha, S. H. Park, S. C. Jung, C. Ryu, J. K. Jeon, M. C. Shin and Y. K. Park, Production and utilization of biochar: A review, J. Ind. Eng. Chem., 2016, 40, 1–15 CrossRef CAS.
- M. B. Ahmed, J. L. Zhou, H. H. Ngo, W. Guo and M. Chen, Progress in the preparation and application of modified biochar for improved contaminant removal from water and wastewater, Bioresour. Technol., 2016, 214, 836–851 CrossRef CAS PubMed.
- S. You, Y. S. Ok, S. S. Chen, D. C. W. Tsang, E. E. Kwon, J. Lee and C.-H. Wang, A critical review on sustainable biochar system through gasification: Energy and environmental applications, Bioresour. Technol., 2017, 246, 242–253 CrossRef CAS PubMed.
- K. Qian, A. Kumar, H. Zhang, D. Bellmer and R. Huhnke, Recent advances in utilization of biochar, Renewable Sustainable Energy Rev., 2015, 42, 1055–1064 CrossRef CAS.
- J. Lee, K. H. Kim and E. E. Kwon, Biochar as a catalyst, Renewable Sustainable Energy Rev., 2017, 77, 70–79 CrossRef CAS.
- D. Fuentes-Cano, A. Gómez-Barea, S. Nilsson and P. Ollero, Decomposition kinetics of model tar compounds over chars with different internal structure to model hot tar removal in biomass gasification, Chem. Eng. J., 2013, 228, 1223–1233 CrossRef CAS.
- S. Hosokai, K. Kumabe, M. Ohshita, K. Norinaga, C.-Z. Li and J.-I. Hayashi, Mechanism of decomposition of aromatics over charcoal and necessary condition for maintaining its activity, Fuel, 2008, 87, 2914–2922 CrossRef CAS.
- T. Matsuhara, S. Hosokai, K. Norinaga, K. Matsuoka, C.-Z. Li and J.-I. Hayashi, Rapid gasification of nascent char in steam atmosphere during the pyrolysis of Na- and Ca-ion-exchanged brown coals in a drop-tube reactor, Energy Fuels, 2009, 24, 76–83 CrossRef.
- S. Hosokai, K. Norinaga, T. Kimura, M. Nakano, C.-Z. Li and J.-I. Hayashi, Reforming of volatiles from the biomass pyrolysis over charcoal in a sequence of coke deposition and steam gasification of coke, Energy Fuels, 2011, 25, 5387–5393 CrossRef CAS.
- T. Sueyasu, T. Oike, A. Mori, S. Kudo, K. Norinaga and J.-I. Hayashi, Simultaneous steam reforming of tar and steam gasification of char from the pyrolysis of potassium-loaded woody biomass, Energy Fuels, 2011, 26, 199–208 CrossRef.
- M. R. Mahishi and D. Y. Goswami, Thermodynamic optimization of biomass gasifier for hydrogen production, Int. J. Hydrogen Energy, 2007, 32, 3831–3840 CrossRef CAS.
- Z. Abu El-Rub, E. Bramer and G. Brem, Experimental comparison of biomass chars with other catalysts for tar reduction, Fuel, 2008, 87, 2243–2252 CrossRef CAS.
- L. Burhenne and T. Aicher, Benzene removal over a fixed bed of wood char: The effect of pyrolysis temperature and activation with CO2 on the char reactivity, Fuel Process. Technol., 2014, 127, 140–148 CrossRef CAS.
- F. Nestler, L. Burhenne, M. J. Amtenbrink and T. Aicher, Catalytic decomposition of biomass tars: The impact of wood char surface characteristics
on the catalytic performance for naphthalene removal, Fuel Process. Technol., 2016, 145, 31–41 CrossRef CAS.
- Y. Zhang, Y. Luo, W. Wu, S. Zhao and Y. Long, Heterogeneous cracking reaction of tar over biomass char, using naphthalene as model biomass tar, Energy Fuels, 2014, 28, 3129–3137 CrossRef CAS.
- P. Gilbert, C. Ryu, V. N. Sharifi and J. Swithenbank, Tar reduction in pyrolysis vapours from biomass over a hot char bed, Bioresour. Technol., 2009, 100, 6045–6051 CrossRef CAS PubMed.
- F. Dabai, N. Paterson, M. Millan, P. Fennell and R. Kandiyoti, Tar formation and destruction in a fixed bed reactor simulating downdraft gasification: equipment development and characterization of tar-cracking products, Energy Fuels, 2010, 24, 4560–4570 CrossRef CAS.
- W. Wu, Y. Luo, Y. Su, Y. Zhang, S. Zhao and Y. Wang, Nascent biomass tar evolution properties under homogeneous/heterogeneous decomposition condition in a two-stage reactor, Energy Fuels, 2011, 25, 5394–5406 CrossRef CAS.
- S. Zhao, Y. Luo, Y. Zhang and Y. Long, Experimental investigation of the synergy effect of partial oxidation and bio-char on biomass tar reduction, J. Anal. Appl. Pyrolysis, 2015, 112, 262–269 CrossRef CAS.
- Y. Zhang, W. Wu, S. Zhao, Y. Long and Y. Luo, Experimental study on pyrolysis tar removal over rice straw char and inner pore structure evolution of char, Fuel Process. Technol., 2015, 134, 333–344 CrossRef CAS.
- A. S. Al-Rahbi, J. A. Onwudili and P. T. Williams, Thermal decomposition and gasification of biomass pyrolysis gases using a hot bed of waste derived pyrolysis char, Bioresour. Technol., 2016, 204, 71–79 CrossRef CAS PubMed.
- J. Park, Y. Lee and C. Ryu, Reduction of primary tar vapor from biomass by hot char particles in fixed bed gasification, Biomass Bioenergy, 2016, 90, 114–121 CrossRef CAS.
- C. Franco, F. Pinto, I. Gulyurtlu and I. Cabrita, The study of reactions influencing the biomass steam gasification process, Fuel, 2003, 82, 835–842 CrossRef CAS.
- J. Gil, J. Corella, M. a. P. Aznar and M. A. Caballero, Biomass gasification in atmospheric and bubbling fluidized bed: effect of the type of gasifying agent on the product distribution, Biomass Bioenergy, 1999, 17, 389–403 CrossRef CAS.
-
C.-Z. Li, Advances in the Science of Victorian Brown Coal, Elsevier, 2004 Search PubMed.
- C.-Z. Li, Importance of volatile-char interactions during the pyrolysis and gasification of low-rank fuels, Fuel, 2013, 112, 609–623 CrossRef CAS.
- P. N. Bhandari, A. Kumar, D. D. Bellmer and R. L. Huhnke, Synthesis and evaluation of biochar-derived catalysts for removal of toluene (model tar) from biomass-generated producer gas, Renewable Energy, 2014, 66, 346–353 CrossRef CAS.
- Q. Huang, P. Lu, B. Hu, Y. Chi and J. Yan, Cracking of model tar species from the gasification of municipal solid waste using commercial and waste-derived catalysts, Energy Fuels, 2016, 30, 5740–5748 CrossRef CAS.
- P. Lu, X. Qian, Q. Huang, Y. Chi and J. Yan, Catalytic cracking of toluene as a tar model compound using sewage-sludge-derived char, Energy Fuels, 2016, 30, 8327–8334 CrossRef CAS.
- P. Lu, Q. Huang, Y. Chi and J. Yan, Preparation of high catalytic activity biochar from biomass waste for tar conversion, J. Anal. Appl. Pyrolysis, 2017, 127, 47–56 CrossRef CAS.
- Z. Ma, R. Xiao and H. Zhang, Catalytic steam reforming of bio-oil model compounds for hydrogen-rich gas production using bio-char as catalyst, Int. J. Hydrogen Energy, 2017, 42, 3579–3585 CrossRef CAS.
- N. Wang, D. Chen, U. Arena and P. He, Hot char-catalytic reforming of volatiles from MSW pyrolysis, Appl. Energy, 2017, 191, 111–124 CrossRef CAS.
- K. Qian and A. Kumar, Reforming of lignin-derived tars over char-based catalyst using Py-GC/MS, Fuel, 2015, 162, 47–54 CrossRef CAS.
- Y. Shen, M. Chen, T. Sun and J. Jia, Catalytic reforming of pyrolysis tar over metallic nickel nanoparticles embedded in pyrochar, Fuel, 2015, 159, 570–579 CrossRef CAS.
- D. Yao, Q. Hu, D. Wang, H. Yang, C. Wu, X. Wang and H. Chen, Hydrogen production from biomass gasification using biochar as a catalyst/support, Bioresour. Technol., 2016, 216, 159–164 CrossRef CAS PubMed.
- J. R. Kastner, S. Mani and A. Juneja, Catalytic decomposition of tar using iron supported biochar, Fuel Process. Technol., 2015, 130, 31–37 CrossRef CAS.
- Y. Wang, L. Jiang, S. Hu, S. Su, Y. Zhou, J. Xiang, S. Zhang and C. Li, Evolution of structure and activity of char-supported iron catalysts prepared for steam reforming of bio-oil, Fuel Process. Technol., 2017, 158, 180–190 CrossRef CAS.
- R. S. Postma, S. R. A. Kersten and G. van Rossum, Potassium-salt-catalyzed tar reduction during pyrolysis oil gasification, Ind. Eng. Chem. Res., 2016, 55, 7226–7230 CrossRef CAS.
- F. Guo, Y. Liu, Y. Liu and C. Guo, Catalytic reforming of tar using corncob char and char-supported potassium catalysts, J. Therm. Anal. Calorim., 2017, 130, 1297–1306 CrossRef CAS.
- J. Ashok and S. Kawi, Steam reforming of toluene as a biomass tar model compound over CeO2 promoted Ni/CaO-Al2O3 catalytic systems, Int. J. Hydrogen Energy, 2013, 38, 13938–13949 CrossRef CAS.
- A. C. C. Chang, L. S. Chang, C. Y. Tsai and Y. C. Chan, Steam reforming of gasification-derived tar for syngas production, Int. J. Hydrogen Energy, 2014, 39, 19376–19381 CrossRef CAS.
- C. Li, D. Hirabayashi and K. Suzuki, Development of new nickel based catalyst for biomass tar steam reforming producing H2-rich syngas, Fuel Process. Technol., 2009, 90, 790–796 CrossRef CAS.
- M. Kaewpanha, G. Guan, Y. Ma, X. Hao, Z. Zhang, P. Reubroychareon, K. Kusaka and A. Abudula, Hydrogen production by steam reforming of biomass tar over biomass char supported molybdenum carbide catalyst, Int. J. Hydrogen Energy, 2015, 40, 7974–7982 CrossRef CAS.
- Y. Shen, Carbothermal synthesis of metal-functionalized nanostructures for energy and environmental applications, J. Mater. Chem. A, 2015, 3, 13114–13188 CAS.
- C. Gai, F. Zhang, Q. Lang, T. Liu, N. Peng and Z. Liu, Facile one-pot synthesis of iron nanoparticles immobilized into the porous hydrochar for catalytic decomposition of phenol, Appl. Catal., B, 2017, 204, 566–576 CrossRef CAS.
- C. Gai, F. Zhang, Y. Guo, N. Peng, T. Liu, Q. Lang, Y. Xia and Z. Liu, Hydrochar-supported, in situ-generated nickel nanoparticles for sorption-enhanced catalytic gasification of sewage sludge, ACS Sustainable Chem. Eng., 2017, 5, 7613–7622 CrossRef CAS.
- Y. Richardson, J. Blin and A. Julbe, A short overview on purification and conditioning of syngas produced by biomass gasification: Catalytic strategies, process intensification and new concepts, Prog. Energy Combust. Sci., 2012, 38, 765–781 CrossRef CAS.
- Y. C. Lin and G. W. Huber, The critical role of heterogeneous catalysis in lignocellulosic biomass conversion, Energy Environ. Sci., 2009, 2, 68–80 CAS.
- C. H. Zhou, X. Xia, C. X. Lin, D. S. Tong and J. Beltramini, Catalytic conversion of lignocellulosic biomass to fine chemicals and fuels, Chem. Soc. Rev., 2011, 40, 5588–5617 RSC.
- V. S. Sikarwar, M. Zhao, P. Clough, J. Yao, X. Zhong, M. Z. Memon, N. Shah, E. J. Anthony and P. S. Fennell, An overview of advances in biomass gasification, Energy Environ. Sci., 2016, 9, 2939–2977 CAS.
- V. S. Sikarwar, M. Zhao, P. S. Fennell, N. Shah and E. J. Anthony, Progress in biofuel production from gasification, Prog. Energy Combust. Sci., 2017, 61, 189–248 CrossRef.
- M. Asadullah, Biomass gasification gas cleaning for downstream applications: A comparative critical review, Renewable Sustainable Energy Rev., 2014, 40, 118–132 CrossRef CAS.
- K. J. Andersson, M. S. S. Rasmussen and P. E. H. Nielsen, Industrial-scale gas conditioning including Topsoe tar reforming and purification downstream biomass gasifiers: An overview and recent examples, Fuel, 2017, 203, 1026–1030 CrossRef CAS.
- L. F. de Diego, F. García-Labiano, P. Gayán, A. Abad, T. Mendiara, J. Adánez, M. Nacken and S. Heidenreich, Tar abatement for clean syngas production during biomass gasification in a dual fluidized bed, Fuel Process. Technol., 2016, 152, 116–123 CrossRef CAS.
- S. N. Reddy, S. Nanda, A. K. Dalai and J. A. Kozinski, Supercritical water gasification of biomass for hydrogen production, Int. J. Hydrogen Energy, 2014, 39, 6912–6926 CrossRef CAS.
- K. Kang, R. Azargohar, A. K. Dalai and H. Wang, Hydrogen production from lignin, cellulose and waste biomass via supercritical water gasification: Catalyst activity and process optimization study, Energy Convers. Manage., 2016, 117, 528–537 CrossRef CAS.
- C. R. Correa and A. Kruse, Supercritical water gasification of biomass for hydrogen production – Review, J. Supercrit. Fluids, 2017 DOI:10.1016/j.supflu.2017.09.019.
- J. Moon, W. Jo, S. Jeong, B. Bang, Y. Choi, J. Hwang and U. Lee, Gas cleaning with molten tin for hydrogen sulfide and tar in producer gas generated from biomass gasification, Energy, 2017, 130, 318–326 CrossRef CAS.
- B. J. Hathaway, M. Honda, D. B. Kittelson and J. H. Davidson, Steam gasification of plant biomass using molten carbonate salts, Energy, 2013, 49, 211–217 CrossRef CAS.
- P. Xiao, L. Guo, X. Zhang, C. Zhu and S. Ma, Continuous hydrogen production by biomass gasification in supercritical water heated by molten salt flow: System development and reactor assessment, Int. J. Hydrogen Energy, 2013, 38, 12927–12937 CrossRef CAS.
- J. Udomsirichakorn and P. A. Salam, Review of hydrogen-enriched gas production from steam gasification of biomass: The prospect of CaO-based chemical looping gasification, Renewable Sustainable Energy Rev., 2014, 30, 565–579 CrossRef CAS.
- X. Zhao, H. Zhou, V. S. Sikarwar, M. Zhao, A.-H. A. Park, P. S. Fennell, L. Shen and L.-S. Fan, Biomass-based chemical looping technologies: the good, the bad and the future, Energy Environ. Sci., 2017, 10, 1885–1910 CAS.
- M. Keller, H. Leion and T. Mattisson, Chemical looping tar reforming using La/Sr/Fe-containing mixed oxides supported on ZrO2, Appl. Catal., B, 2016, 183, 298–307 CrossRef CAS.
- Y. Richardson, J. Motuzas, A. Julbe, G. Volle and J. Blin, Catalytic investigation of in situ generated Ni metal nanoparticles for tar conversion during biomass pyrolysis, J. Phys. Chem. C, 2013, 117, 23812–23831 CAS.
- Y. Shen and K. Yoshikawa, Tar conversion and vapor upgrading via in situ catalysis using silica-based nickel nanoparticles embedded in rice husk char for biomass pyrolysis/gasification, Ind. Eng. Chem. Res., 2014, 53, 10929–10942 CrossRef CAS.
- Y. Shen, P. Zhao, Q. Shao, F. Takahashi and K. Yoshikawa, In situ catalytic conversion of tar using rice husk char/ash supported nickel–iron catalysts for biomass pyrolytic gasification combined with the mixing-simulation in fluidized-bed gasifier, Appl. Energy, 2015, 160, 808–819 CrossRef CAS.
- Y. Shen, M. Ding, X. Ge and M. Chen, Catalytic CO2 gasification of rice husk char for syngas and silica-based nickel nanoparticles production, Ind. Eng. Chem. Res., 2015, 54, 8919–8928 CrossRef CAS.
- Y. Liu, F. Guo, X. Li, T. Li, K. Peng and C. Guo, Catalytic effect of iron and nickel on gas formation from fast biomass pyrolysis in a microfluidized bed reactor: A kinetic study, Energy Fuels, 2017, 31, 12278–12287 CrossRef CAS.
- J. Xue, G. Dou, E. Ziade and J. L. Goldfard, Integrating sustainable biofuel and silver nanomaterial production for in situ upgrading of cellulosic biomass pyrolysis, Energy Convers. Manage., 2017, 142, 143–152 CrossRef CAS.
- Z. Liu, P. McNamara and D. Zitomer, Autocatalytic pyrolysis of wastewater biosolids for product upgrading, Environ. Sci. Technol., 2017, 51, 9808–9816 CrossRef CAS PubMed.
- J. A. Bennett, K. Wilson and A. F. Lee, Catalytic applications of waste derived materials, J. Mater. Chem. A, 2016, 4, 3617–3637 CAS.
- D. Wang, W. Yuan and W. Ji, Char and char-supported nickel catalysts for secondary syngas cleanup and conditioning, Appl. Energy, 2011, 88, 1656–1663 CrossRef CAS.
- G. Yu, Y. Feng, D. Chen, M. Yang, T. Yu and X. Dai, In situ reforming of the volatile by char during sewage sludge pyrolysis, Energy Fuels, 2016, 30, 10396–10403 CrossRef CAS.
- G. Yu, D. Chen, U. Arena, Z. Huang and X. Dai, Reforming sewage sludge pyrolysis volatile with Fe-embedded char: Minimization of liquid product yield, Waste Manage., 2017 DOI:10.1016/j.wasman.2017.08.004.
- Y. Song, Y. Wang, X. Hu, S. Hu, J. Xiang, L. Zhang, S. Zhang, Z. Min and C. Li, Effects of volatile-char interactions on in situ destruction of nascent tar during the pyrolysis and gasification of biomass. Part I. Roles of nascent char, Fuel, 2014, 122, 60–66 CrossRef CAS.
- Y. Song, Y. Wang, X. Hu, J. Xiang, S. Hu, D. Mourant, T. Li, L. Wu and C. Li, Effects of volatile–char interactions on in-situ destruction of nascent tar during the pyrolysis and gasification of biomass. Part II. Roles of steam, Fuel, 2015, 143, 555–562 CrossRef CAS.
- Y. Song, J. Xiang, S. Hu, D. M. Quyn, Y. Zhao, X. Hu, Y. Wang and C. Li, Importance of the aromatic structures in volatiles to the in-situ destruction of nascent tar during the volatile–char interactions, Fuel Process. Technol., 2015, 132, 31–38 CrossRef CAS.
- D. Feng, Y. Zhao, Y. Zhang, Z. Zhang, H. Che and S. Sun, Experimental comparison of biochar species on in-situ biomass tar H2O reforming over biochar, Int. J. Hydrogen Energy, 2017, 42, 24035–24046 CrossRef CAS.
- D. Feng, Y. Zhao, Y. Zhang, Z. Zhang and S. Sun, Roles and fates of K and Ca species on biochar structure during in-situ tar H2O reforming over nascent biochar, Int. J. Hydrogen Energy, 2017, 42, 21686–21696 CrossRef CAS.
- D. Feng, Y. Zhao, Y. Zhang, S. Sun, S. Meng, Y. Guo and Y. Huang, Effects of K and Ca on reforming of model tar compounds with pyrolysis biochars under H2O or CO2, Chem. Eng. J., 2016, 306, 422–432 CrossRef CAS.
- D. Feng, Y. Zhao, Y. Zhang, J. Gao and S. Sun, Changes of biochar physiochemical structures during tar H2O and CO2 heterogeneous reforming with biochar, Fuel Process. Technol., 2017, 165, 72–79 CrossRef CAS.
- D. Feng, Y. Zhao, Y. Zhang and S. Sun, Effect of H2O and CO2 on the homogeneous conversion and heterogeneous \orming of biomass tar over biochar, Int. J. Hydrogen Energy, 2017, 42, 13070–13084 CrossRef CAS.
- C. Z. Li, C. Sathe, J. R. Kershaw and Y. Pang, Fates and roles of alkali and alkaline earth metals during the pyrolysis of a Victorian brown coal, Fuel, 2000, 79, 427–438 CrossRef CAS.
- C. Sathe, Y. Pang and C.-Z. Li, Effects of heating rate and ion-exchangeable cations on the pyrolysis yields from a Victorian brown coal, Energy Fuels, 1999, 13, 748–755 CrossRef CAS.
- C.-Z. Li, Some recent advances in the understanding of the pyrolysis and gasification behaviour of Victorian brown coal, Fuel, 2007, 86, 1664–1683 CrossRef CAS.
- C. Wu, V. L. Budarin, M. Wang, V. Sharifi, M. J. Gronnow, Y. Wu, J. Swithenbank, J. H. Clark and P. T. Williams, CO2 gasification of bio-char derived from conventional and microwave pyrolysis, Appl. Energy, 2015, 157, 533–539 CrossRef CAS.
- D. Feng, Y. Zhao, Y. Zhang, H. Xu, L. Zhang and S. Sun, Catalytic mechanism of ion-exchanging alkali and alkaline earth metallic species on biochar reactivity during CO2/H2O, Fuel, 2018, 212, 523–532 CrossRef CAS.
|
| This journal is © The Royal Society of Chemistry 2018 |
 *ab and
Yuhong
Fu
a
*ab and
Yuhong
Fu
a












