Catalytic cracking of triglycerides with a base catalyst and modification of pyrolytic oils for production of aviation fuels†
Abstract
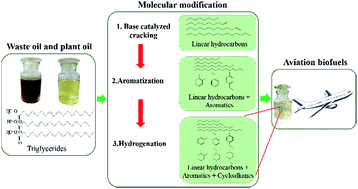
Molecular structure transformation is a critical step for synthesis of aviation biofuels from triglycerides, to yield alkanes, aromatics and cycloalkanes. To this end, triglycerides were treated by catalytic cracking coupled with rectification to obtain hydrocarbon fuels, and the molecular weight of the liquid product was adjusted to the desirable boiling range of aviation fuel. To further modify the molecular structures, the pyrolytic hydrocarbons were partially converted into aromatics and cycloalkanes by a two-step process. Different raw materials including soybean oil, rubber seed oil, waste cooking oil and acidified oil were cracked with a 5 wt% base catalyst at 350–450 °C (under the atmospheric pressure) in a benchtop reactor. Afterwards, the linear hydrocarbons of C8–C15 size were transformed into aromatics by HZSM-5 at 350 °C for 6 h. Then, a portion of the aromatics were converted into cycloalkanes catalyzed by Pd/AC at 200 °C and 6 MPa (H2) for 6 h. As a result, a desirable mixture of linear hydrocarbons, aromatics and cycloalkanes was obtained with a similar composition and properties to traditional fossil aviation fuels.
- This article is part of the themed collection: 2018 Sustainable Energy and Fuels HOT Articles


 Please wait while we load your content...
Please wait while we load your content...