Emerging microalgae technology: a review
S. C.
Pierobon†
 ,
X.
Cheng†
,
X.
Cheng†
 ,
P. J.
Graham†
,
P. J.
Graham†
 ,
B.
Nguyen†
,
B.
Nguyen†
 ,
E. G.
Karakolis†
,
E. G.
Karakolis†
 and
D.
Sinton
and
D.
Sinton
 *
*
Department of Mechanical and Industrial Engineering, Institute for Sustainable Energy, University of Toronto, 5 King's College Road, Toronto, ON M5S 3G8, Canada. E-mail: sinton@mie.utoronto.ca
First published on 27th July 2017
Abstract
Cultivating microalgae has the potential to produce biofuels and bioproducts from solar energy with low land use and without competing with food crops. However, despite these key advantages, barriers remain to widespread, economical production from microalgae including: the relatively low solar energy conversion efficiency of photosynthesis, unknown optimal cultivation conditions, and the high energy and economic costs of cultivation and processing microalgal biomass. Thus, recent technological developments seek to address these barriers. To optimize cultivation conditions, devices taking advantage of advanced fluid and light handling techniques are being developed. These approaches drastically increase experimental throughput to find the ideal cultivation parameters. To apply optimal conditions, a range of cultivation approaches are being developed to deliver light and nutrients to microalgae to achieve high productivities. Finally, to extract maximal value out of microalgal biomass, downstream processing technology, such as hydrothermal liquefaction, is replacing costly conventional processes to produce fuels and high-value products from microalgae. Taken together, these technologies can allow microalgae to become competitive in the sustainable energy landscape – particularly to produce complex, high-value molecules.
1 Introduction
Addressing the future energy and climate concerns will require a diverse set of solutions, including low-carbon energy production, energy storage and carbon capture. Liquid fuels are integral for transportation owing to their high energy density and compatibility with existing transportation infrastructure.1,2 Photosynthesis can drive biological and synthetic processes to create complex molecules including biofuels that can substitute for long-chain fuel hydrocarbons which make up the primary energy source of greatest global demand.1,2 Moreover, microalgae are capable of producing high-value products for example, pigments, proteins and nutraceuticals.3,4Microalgae have advantages as biofuel feedstocks in terms of reduced land-use and do not compete directly with food as with crop-based fuels.5,6 However, despite these advantages biofuel production from algae still faces serious challenges: (1) poor efficiency of photosynthetic solar energy capture;1 (2) sub-optimal culture conditions;1 and (3) high energy and economic costs of cultivation and downstream processing.7–10 New technology will be required to address these challenges. First, lab-based multi-parameter optimization is required to determine the ideal conditions for microalgae cultivation. For this to occur, technology and techniques to explore the extensive range of possible cultivation conditions is required. Secondly, once ideal cultivation conditions are determined photobioreactor technology must be advanced to ensure that those cultivation conditions can be applied uniformly and at reasonable capital and operating cost. Finally, once microalgae are grown efficiently, processing techniques are required to maximize extracted value while minimizing energy input. A number of quality reviews detail the current practices in microalgal cultivation, as well as niche developments.11–14 However, in recent years, key advances have been made in the development of novel and promising technologies pertaining to all aspects of biofuel and bioproduct production from microalgae. In this review, we detail these emerging technologies relevant to microalgae production and processing, and discuss the gaps going forward.
In this review, we overview emerging technologies in the realm of microalgal biotechnology. First, tools to screen for optimal photobioreactor conditions and techniques to implement this knowledge are presented. Next, we will discuss the light and carbon utilization considerations to be made while designing a photobioreactor, which leads into a discussion on porous substrate bioreactors – an emerging design. Lastly, downstream processing techniques will be discussed while highlighting emerging technologies for concentrating, extracting and upgrading microalgal biomass.
2 Informing microalgal production
2.1 The complexity of microalgal photosynthesis
Microalgal productivity is dependent on a variety of parameters, namely temperature, the light characteristics, the aqueous environment, the gaseous environment, and the interactions between these variables. High throughput screening devices, particularly microfluidic devices, present an opportunity to rapidly explore the parameter space associated with microalgal productivity. Microfluidic devices for multiplexed studies of cell biology have already been developed and reviewed extensively.15–17 However, such devices are aimed at studying the role of the chemical environment, typically drugs. In contrast, platforms capable of studying photosynthetic organisms should be capable of multiplexing notably different variables, light, temperatures and dissolved gas concentrations. Thus, new screening platforms have been developed over recent years.18–20 Moreover, implementing this data can involve either direct operating conditions, or predictive models to be used with full scale modeling of the reactor.21–25Aspects of the light environment to be studied include the intensity, spectral distribution and time variance.26 Briefly, increasing light intensity increases growth until a saturating intensity, where other nutrients are the limiting factor and additional light does not increase growth. A further increase in irradiance can become harmful to microalgae due to excess reactive oxygen species (ROS) production.27 The light spectrum plays a role, depending on a photon's wavelength it can either drive photosynthetic growth, if absorbed by pigments associated with the photosynthetic apparatus, or induce signal transduction.28–33 Lastly, the time variance of light plays an important role, where under high frequency fluctuations, photosynthesis occurs at the same rate it would under the equivalent time-averaged irradiance, due to a mismatch in reaction rates.34–43 In short, multiple aspects of the light and chemical environment require comprehensive study and there is a growing need for the associated experimental tools.
2.2 Multiplexed screening of microalgae
Devices capable of multiplexing the light environment have been recently developed.14,20,44–46 These devices multiplex light environments using a diverse set of methods including individual light sources,47 fluid handling to act as neutral density filters,48,49 liquid crystal displays,19,20 and recently, photonic cavities46 (Fig. 1). The methods typically involve adapting an optical device to a microfluidic cell culture chamber. The short optical path of the cell culture chamber allows for a relatively constant light intensity through the depth, improving the accuracy of results. In contrast, flask-scale (10–1000 mL) studies of microalgal growth are obscured due to gradients of light intensity stemming from long optical paths, particularly at higher cell densities.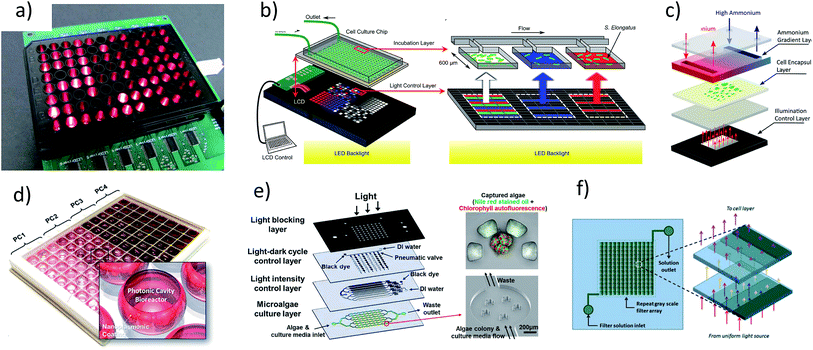 | ||
| Fig. 1 Summary of high throughput devices for microalagal screening: devices for creating multiplexed optical environments. (a) LED based approach.47 (b and c) LCD based approach.19,20 (d) Use of photonic cavities.46 (e and f) Dye based approaches.48,49 Images (a–c, e and f) have been reproduce from ref. 47, 19, 20, 48 and 49 with permission from The Royal Society of Chemistry. Image (d), reprinted with permission from, Integrated Microalgae Analysis Photobioreactor for Rapid Strain Selection, Soon Gweon Hong, Minsun Song, Sungjun Kim, Doyeon Bang, Taewook Kang, Inhee Choi, and Luke P. Lee, ACS Nano, 2016, 10(6), 5635–5642, DOI: 10.1021/acsnano.6b00803. Copyright 2017 American Chemical Society. | ||
However, none of these techniques provide the ability to simultaneously control the spectrum, irradiance, and time variance of the light environment. Light emitting diode (LED) arrays and liquid crystal displays (LCD) provide good control over irradiance and time variance with the ability to adjust the relative intensity of three sections of the spectrum (corresponding to the red, green and blue sections of the visible spectrum). Fluid handling techniques of dyes (Fig. 1e and f)48,49 can in principle be used to control the spectrum, provided the dye or combination of dyes are available, however these methods are likely complex and expensive to fabricate. Moreover, they are also subject to issues of stability related to maintaining well balanced fluid flow of dyes when making gradients and have a slow response time. For example, Kim et al.48 designed a device which could switch from light to dark in two minutes at best, which is much too slow to mimic mixing effects. A more robust set of light intensities using dyes was shown by Lou at al.49 where channels of various heights are used to create multiple irradiance intensities, the benefit of robustness in this approach comes at the price of flexibility.
The use of plasmonics46 provides an academically interesting technique to control local irradiance intensity, but does not have any practical advantages over the use of dyes, LEDs or LCDs. Moreover, coping with the difficulty of characterizing the highly localized light environment in a plasmonic device is likely to be intimidating and not of interest to biologists. One possible exception is those that are interested in illuminating only a portion of a cell. However, this is more easily accomplished adapting current microscopy techniques (e.g. fluorescence recovery after photobleaching).29,50 Phototaxis experiments have been performed by integrating projectors (specifically spatial light modulators) into the optical path of a microscope,51,52 devices of a similar design may be useful for high throughput heavily controlled screening, by integrating multiple light sources, similar to a laser scanning confocal microscope.
Photosynthetic organisms use CO2 for growth, and thus can serve as a means of carbon capture. However, the CO2 response of microalgae is complex, due to its role as a source of growth, acidification and the presence of the carbon concentrating mechanics. Therefore multiplexing the CO2 concentration would be of value, particularly in conjunction with the light environment. A key area where multiplexing is lacking is in gas concentrations. Multiplexing the gas environment is distinct from the chemical environment, since the chemical environment can easily be adjusted by pipetting, including the use of automated pipetting systems. In addition, diffusion-based gradient generators have primarily developed for aqueous chemicals,19,53 and some gas diffusion devices have also been realized.54 However, these devices have been limited to under 10 levels. A large-scale gas multiplexing apparatus has yet to be demonstrated and would be greatly beneficial.
Optimizing the production of high-value bioproducts is key to strengthening the business case for microalgae cultivation.55 In addition, biomanufacturing can be seen as a means to lower the capital cost of chemical synthesis, which can have a disruptive market effect.56 Typically, the accumulation of desired bioproducts depends on environmental conditions. Specifically, stressful conditions such as excess light, high salinity, and nutrient stress often lead to the hyperaccumulation of valuable bioproducts. Maximizing productivity is a balance between biomass generation, bioproduct accumulation, and cell viability.57,58 These complicating parameters mean that techniques to maximize experimental throughput are essential to mapping out the entire parameter space and identifying optimum productivity conditions. The previously mentioned screening platforms can be used to provide a diverse set of environmental parameters, however the readout is typically limited to cell density, or at best a fluorescent signal associated with lipid production. Unfortunately, the low sample volumes of microfluidic systems present an obstacle since most analytical chemical devices require volumes on the order of milliliters, whereas a microfluidic photobioreactor chamber rarely exceeds 100 nL. This presents an opportunity for improving the integration of on-chip measurement techniques such as Raman, and IR spectroscopy. Moreover, the integrated sample prep possible with centrifugal microfluidics or similar micro total analysis systems.59
2.3 Scaling up knowledge
Transferring the light and chemical information from multiplexed studies to the design of a photobioreactor is more complex than matching the parameters of the reactor to the lab data, due to the heterogeneity of conditions within the reactor – specifically gradients in light intensity and chemical concentration. Our recent work highlights the possible disconnect between small scale information and reactor scale data, by demonstrating that poorly absorbed green light is more effective than well absorbed red light at intensities when running a high density photobioreactor.60 The improved performance occurs due to a more uniform light distribution through the reactor. Being more representative of the light environment of a given microalgal cell, data from short optical that reactors (reactors where cells receive a similar irradiance intensity) should be used in conjunction with details regarding the large-scale gradients in full-scale photobioreactors.Due to the combination of light gradients and mixing, microalgae in a large scale photobioreactor, receive a time varied irradiance.34,42,43,61,62 As mentioned earlier, the growth of microalgae is dependent on the time history of the light it receives (Fig. 2a). Detailed design of a photobioreactor would require a model that is not too heavy computationally, and could be used in conjunction with a CFD (computational fluid dynamics) code to track macroalgal trajectories.21,22,24,63,64 To address this, multiple simple two or three state models have been developed (Fig. 2b and c).34,42,61,62 Generally, these models simplify the major photosynthetic reactions into a fast set of reactions and a slow set of reactions. Due to the mismatch in rates, there is a buildup of intermediate products to allow growth in the dark, and such dynamic light effects can be captured through suitable models.
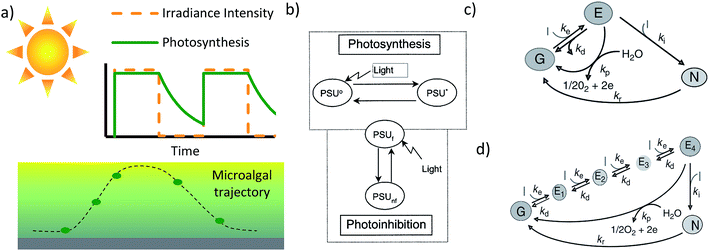 | ||
| Fig. 2 (a) Schematic illustrating a modelled photosynthetic response to a light profile experienced in a microalgal culture. (b) Three state model of photosynthesis, including the photosystem as being either resting, excited or inhibited. The decay from excited to resting dictates the growth rate. In this case the excitation from resting to excited state corresponds to the entire photosynthetic electron transport chain42,65–67 Reprinted from Journal of Theoretical Biology, 304, F. García-Camacho, A. Sánchez-Mirón, E. Molina-Grima, F. Camacho-Rubio, J. C. Merchuck, a mechanistic model of photosynthesis in microalgae including photoacclimation dynamics, 1, Copyright 2012, with permission from Elsevier. (c) A similar three state model, however the excitation from the ground state to the excited state relates to exciting photosystem II and the recovery step relates to water splitting. (d) A model similar to the model in (c), but taking into account that multiple steps are required to excite photosystem II. Images (c) and (d) have been reproduced from Handbook of Microalgal Culture: Applied Phycology and Biotechnology, Second Edition, Chapter 12: the essential role of timescales. Copyright Wiley 2013. | ||
The bulk of the models simplify photosynthesis by considering the light harvesting apparatus and the electron transport chain as a single Photosynthetic Unit (PSU), (Fig. 2b). Light drives the excitation of the photosynthetic unit, where the excited state corresponds to the cell having an increased concentration of ATP and NADPH. The ATP and NADPH are then used during the de-excitation process which represents the Calvin cycle. Moreover, these models have also captured the effect of photoinhibition. This simplified model has been used to capture growth in flashing light, where at a sufficiently high frequency (>5 Hz) the growth rate can reach the growth rate possible under the equivalent time-average irradiance.42,65–67 A photosynthetic unit can also become inhibited should it receive an excessively high irradiance for a sustained period. In contrast to the PSU style model, models from Zarmi and colleagues25,68–70 (Fig. 2c and d) relate to the mismatch between reactions along the photosynthetic electron transport chain. Though progress has been made in developing useable models, integrating all the relevant timescales associated with photosynthetic growth and induction timescales is required.
2.4 Genetic engineering and strain selection
Genetic engineering and strain selection can benefit from the fluid handling and automation associated with microfluidic techniques (Fig. 3). Digital microfluidics has shown promise in the reduction of labor associated with genetic engineering as well as savings on costly reagents.71,72 Strain selection methods have been improved using droplet microfluidics extensively.73 In addition, recent devices have emerged that leverage correlations between buoyancy and lipid content74 as well as phototaxis and photosynthetic growth.75 Tailoring strain selection and genetic engineering tools to be effective for microalgal screening would accelerate microalgal implementation.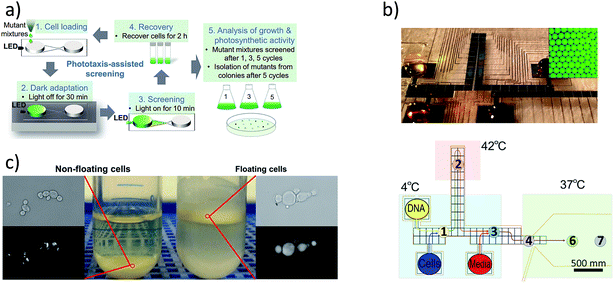 | ||
| Fig. 3 (a) Strain selection based on improved phototactic response,75 (b) gene manipulation using droplet microfluidics,73 reprinted with permission from Shih et al.72 Copyright 2015 American Chemical Society, and (c) selection of high lipid producing yeast using buoyancy.74 Reprinted from Metabolic Engineering, 29, Leqian Liu, Anny Pan, Caitlin Spofford, Nijia Zhou, Hal S. Alper, an evolutionary metabolic engineering approach for enhancing lipogenesis in Yarrowia lipolytica, 36–45, Copyright 2015, with permission from Elsevier. | ||
2.5 Environmental applications
In the context of this review, understanding the interactive effects of multiple variables has been useful for identifying conditions with high microalgal productivity, however this endeavor is also of great interest and importance in broader ecological contexts. The need for multiparameter control when cultivating microalgae mirrors the need to understand influential parameters in the environmental context. However, the motivation to decouple the interactive effects of environmental parameters in ecological settings, in contrast to microalgal studies, stems from the need to identify detrimental combinations that cause harmful effects to global ecosystems as opposed to those which maximize productivity.The natural environment is impacted simultaneously by both anthropogenic climate change and ecotoxic pollution. The onset of warming,76 CO2 driven acidification77 and increased ultraviolet B radiation (UVB),78 in conjunction with chemical pollutants79,80 act as stressors to global ecosystems. As with microalgae growth parameters, the effect of multiple anthropogenic stressors cannot always be inferred from studying constituent parameters independently due to complicated interactive effects.81–83 Furthermore, the increased complexity of macroorganisms and the interactions between them in ecosystems makes it even more difficult to decouple the effects of system variables. As a result, an effective assessment of multiple stressors requires studying all relevant stressors in parallel, with many different combinations83,84 (Fig. 4).
Methods, devices and techniques have been developed to achieve precise control over relevant stressor parameters in ecological studies, however the experimental throughput challenge,81 also present with microalgae cultivation optimization, has not been addressed. Commonly studied aquatic stressors include: CO2-driven acidification, increased UVB radiation and warming. Acidification is typically controlled by injecting air/CO2 mixtures into the headspace of a testing chamber85 (Fig. 5b), bubbling into aqueous solution85–88 (Fig. 5b and d), adding CO2 saturated media89–91 (Fig. 5a, e and f) or adjusting pH directly92 (Fig. 5g); Ultraviolet B radiation by either UVB lamps93,94 or a combination of natural sunlight and filters;95,96 and warming through actively heating the system (e.g. immersion water heaters97) (Fig. 5c–e) or passively warming, via enhancement of natural warming.98 Devices and systems have been developed to incorporate control over combinations of these parameters for multiplexed studies,88,99,100 however, the majority are large and costly, limiting the combinations and replicates that can be feasibly achieved. In general, these devices achieve control over relevant parameters generating environments capable of simple multi-stressor analysis (varying 1–2 stressors) but fall short in their inability to feasibly study sufficient combinations – with replicates – to decouple the effects of complex stressor interactions (varying 4+ stressors). That is, full factorial experiments are ideal in terms of predictive power and mechanistic insight.101 Tradition systems are practically limited to under 100 experimental units (Fig. 5) – not enough to perform multi-parameter full factorial experiments of relevant stressors.
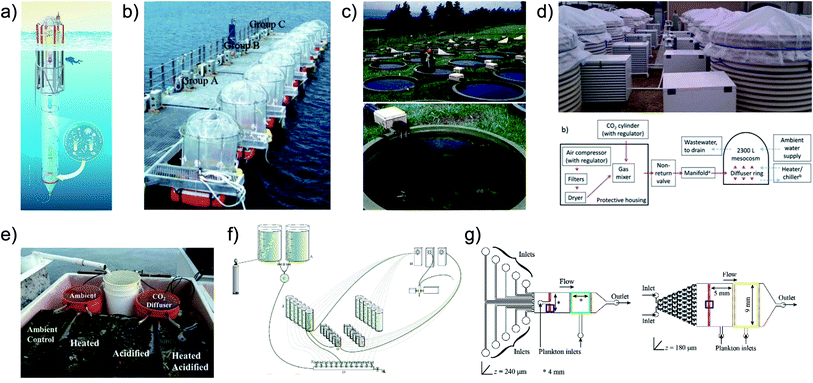 | ||
| Fig. 5 Summary of some existing techniques for studying multiple stressors in controlled environments. (a and b) Large scale offshore devices controlling CO2 levels while leveraging actual sea temperature and light conditions.85,90 (c and d) Large scale on-shore systems with varying levels of control. (c) Aqueous and temperature control, but with natural light.97 (d) Aqueous and temperature control.88 (e and f) Small scale systems. (e) Aqueous and temperature control with natural light.91 (f) Aqueous control.89 (g) Microscale system with control of aqueous variables.92 Image (a) is reproduced under the Creative Commons Attribution License [http://https://creativecommons.org/licenses/by/3.0/]. Images (b) and (d–f) have been reproduced with permission from Wiley Periodicals. Image (c) is reproduced with permission from the Freshwater Biological Association. Image (g) is reproduced under the Creative Commons Attribution License [http://https://creativecommons.org/licenses/by/4.0/]. | ||
The microalgae technology reviewed here are compatible with ecologically relevant microalgal species and marine microbes84 and in contrast to the existing ecological techniques, are capable of generating sufficient combinations and replicates necessary for the multi-stressor challenge. Microalgae alone are responsible for about half of global primary productivity,102 however despite their importance to the carbon cycle, questions remain about how microalgae will respond to future environmental conditions. Particularly, growth responses to increasing CO2 and temperature in a diverse set of local environments has broad implications for global carbon fixation and ecosystem health. Beyond microorganisms, we see potential for some of the technology reviewed here to be scaled for larger, more complex organisms and systems (Fig. 6). That being said, the strengths of these technologies, namely small volumes and precise control of parameters, limit the scope that can be studied to simple mechanistic biological effects.103 This is because in ecology and ecotoxicology studies, a tradeoff between control and realism exists, and depending on the complexity of the system and response being studied, a larger more realistic system with less control might be necessary.104 For instance, a comprehensive study of ecosystem responses to eutrophication105 would not be possible with a small-scale highly controlled approach and would instead require larger volumes with multiple species of organisms to replicate complex ecosystem interactions. However, studying the effects of acidification, warming, increased UVB radiation and pollutant exposure on the larval development and growth of a single organism or small assemblage of organisms, is possible and well suited to this approach. Overall, we see diverse opportunities here for both emerging technologies in microalgae production, and in broader environmental application, in the development of more advanced microcosms uniquely tailored to multi-stressor ecological experiments.
3 Optimizing the photobioreactor
3.1 Major photobioreactor production factors
Photobioreactor optimization, driven by the promise of cost-effective biofuel production, is an iterative process of proving laboratory-scale production potential and evaluating pilot plant cost performance of proven designs. Several recent techno-economic and lifetime assessment studies using pilot plant data from conventional reactor designs (Fig. 7) together suggest a microalgae oil price point of 0.3–8$ US per L (ref. 55 and 106–109) two orders of magnitude lower than the price predicted two decades ago,110 and not far from the current conventional diesel market price of ∼0.7$ US per L.111 The validation of assessment models toward reducing price point uncertainties is complicated by the rarity of consistent, comparable pilot plant data107 and models that sometimes inconsistently define system boundaries and make questionable assumptions.112 However, assessment studies have consistently identified a set of production cost and productivity factors that inform photobioreactor optimization. The major production cost factors include labor, infrastructure, operational energy, product extraction, carbon supply and – depending on species demand – micronutrient supply (namely, nitrogen and phosphorous).106–108,112–116 The relative size of these costs depends on the strategies employed as well as operation-specific parameters. The most important productivity factors are light and carbon.1,55,112,115,117–121 The remainder of this section summarizes reactor design approaches for efficient light and carbon utilization while mitigating major production costs. This section concludes with an overview of unconventional reactor designs with novel means to improve productivity.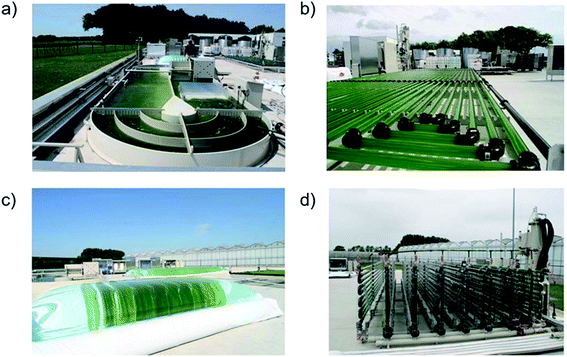 | ||
| Fig. 7 Pilot plant (a) raceway pond (b) horizontal tubular (c) bag-style flat panel (d) vertical tubular photobioreactors in operation at AlgaePARC, Wageningen UR, the Netherlands (modified from ref. 120. Reproduced under the Creative Commons Attribution License [http://https://creativecommons.org/licenses/by/4.0/]). | ||
3.2 Light distribution
Efficient light utilization by cultures requires the intensity extremes in the reactor to span the optimal photosynthetic range for the species.55,120,122,123 Conventional reactors that optimally use sunlight at ∼2000 μmol m−2 s−1 of 400–700 nm photosynthetically active radiation (PAR) must dilute captured intensities about ten times before transmission through a culture thickness that defines the optimal low-intensity.124,125 The spatial aspect ratios of flat panel reactors are ideal for sunlight dilution by capture-to-ground areas, and limit intensities by culture-volume-to-capture areas in ‘short light path’ designs (∼cm).120,126 Flat panel reactors achieve the greatest biomass productivities and photosynthetic efficiencies of conventional designs55,68,120,123,124,127–129 though the necessary 1–10 cm gaps between adjacent panels that enables significant capture of diffuse light (∼10–40% of solar radiation on clear days130) also limits facility areal density.55,120,123,126 Closely stacked flat panels enable complete facility area usage, with compartment walls that can serve to effectively distribute light,131,132 though additional infrastructure is required for external light collection and concentration.Light distribution by planar waveguides or ‘light guides’ between flat panel compartments enable similar intensity control as conventional flat panel reactors, with spatial dilution ratios of input-to-emission surface areas and low-intensities defined by inter-waveguide spacing129,133 (Fig. 8a). Optimal uniform emission from waveguides occurs by breaking total internal reflection, which occurs at sub-critical angles by means of tapering angle or a lengthwise distribution of internal or surface scatterers.134–140 Uniform emission at large supercritical angles is also possible by means of frustrated total internal reflection, induced by biomass contact with near-surface evanescent fields on unmodified waveguide surfaces.141–143 Planar-stack waveguide reactors employing micro-patterned waveguide surfaces133 and internal light-scattering particles134 have exceeded equivalent flat panel volumetric biomass productivities by 3–8× and densities by 3–4× even without control of the low-intensity limit, owing to the greater and more uniform illumination areas. Additionally, illumination intensity and distribution can be enhanced by modifying waveguide optical properties, as demonstrated with added broadband134,144 or wavelength-selective145 reflectors on waveguides, and waveguide integration with spectrum-converting luminescent particles146–151 or sensitizer–emitter pairs.152 Conversion of unproductive solar wavelengths to green is particularly beneficial for high-density production in suspension cultures due to greater light dilution achievable at low cell-absorption wavelengths, as demonstrated by Ooms et al. (2016)60 (Fig. 8b). In contrast, solar wavelengths outside the PAR range may be captured by photovoltaics, as demonstrated in a biomass and electricity co-generation reactor.153 Effective utilization of the solar spectrum with the above approaches may also be combined with co-cultivation of microalgae with complementary absorbance spectra.154,155 Recent novel light distribution methods with less control over intensities relative to the above approaches include light-scattering open-pore glass sponges (Fig. 8c)156,157 and externally powered wireless LEDs in suspension.158 The reactor volume occupied by such internal emitter should be minimalized to ensure a high-density facility and mitigate infrastructure costs.143,159
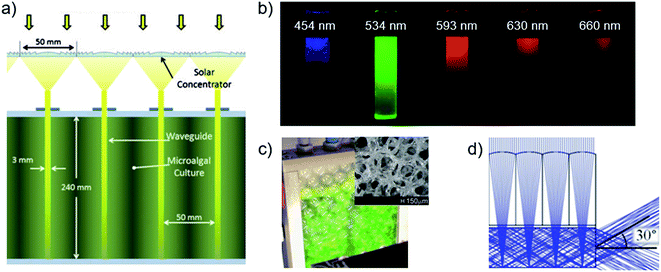 | ||
| Fig. 8 Internal light management strategies in photobioreactors. (a) Fresnel lenses concentrate and directly couple sunlight into waveguides.165 (b) Photographs of columns of cyanobacteria suspensions visualizing the penetration depth of different wavelengths.60 (c) Photobioreactor using glass sponge (structure inset) to distribute light.156 (d) Ray-tracing in a planar solar concentrator.168 Image (a) is reproduced with permission from the American Society of Mechanical Engineers. Image (b) is reproduced with permission from Wiley Periodicals. Images (c and d) are reproduced under the Creative Commons Attribution License [http://https://creativecommons.org/licenses/by/4.0/]. | ||
Despite lab-scale demonstrations of the numerous illumination-based production advantages offered by stacked-waveguide reactors, pilot plant investigations have not been realized, partly by difficulties in achieving a cost-effective external solar concentrator and light delivery system. Systems that use multiple and disparate optical elements demonstrate prohibitively large losses >50% (typically using primary Fresnel lenses and secondary lenses or optical fibers).138,160–164 Chained losses from reflection, absorption, aberration and unwanted breaking of total internal reflection are minimized by directly coupling concentration and dilution elements that are optically similar, for example, in consideration of acceptance angle and f/# focal ratio.125,162,165 In addition, to enable cost-effective production of low-value biomass, high costs of active solar tracking should be avoided with solar concentration factors optimized to large acceptance angles.129,162,163,166 Dye et al. (2011) developed a minimal optical system with ∼70% optical efficiency using 1-D Fresnel lenses that concentrate light directly into stacked reactor waveguides (Fig. 8a), though with active tracking.165 Planar solar concentrators, recently developed for photovoltaic applications, contain many spherical micro-optical lens–mirror pairs that have achieved overall >80% optical efficiencies at concentration factors ≲100× but with narrow acceptance angels (<1°) (Fig. 8d).167–169 The implementation cylindrical lenses and/or active refractive index materials may improve acceptance angles to ±50° East-West and ±10° North-South.170,171 The high physical and optical compatibility of planar solar concentrators with light-diluting planar waveguides may then enable cost-effective solar stacked-waveguide reactors for biomass production.172 Further considerations for light-management strategies in photobioreactors and associated efficiencies, may be found in the recent review by Ooms, et al. (2016).1
3.3 Carbon distribution
Efficient carbon utilization occurs in reactors that closely match the supply rate of dissolved carbon with culture demand.173 In reactors that maintain optimal pH, the carbon supply rate is mainly a function of the CO2(g) aeration rate, concentration, and overall carbon mass transfer coefficient of the reactor (KL), which is dominated by that of the liquid film diffusion barrier at the gas–liquid interface (KLa).174–176 In conventional reactors, aeration is accomplished using macrobubbles (1–2 mm diameter) with low KLa that necessitate elevated CO2 concentrations and long bubble residence times to meet culture demand.117,177,178 On-demand injection of elevated CO2 in (closed) reactors then results in greater carbon utilization efficiencies (50–96%)117,179–181 than with continuous aeration (<10%)177,182 due to the elevated concentration lost to atmosphere. By increasing continuous aeration flow rate, or KLa with higher-pressure bubbles, productivity is improved at greater operational energy cost and much reduced carbon utilization efficiency.117,121,177,178,183 However, to reduce carbon supply cost when an inexpensive, high-concentration source (i.e. flue gas) is not available, purchased CO2(g) supply rate must be lowered, which necessitates continuous aeration to meet culture demand. Production and cost considerations using carbon supplied from flue gas are reviewed in ref. 181 and 184–187. With CO2 supply concentrations less than the 1–5% typically used in laboratory and pilot plant reactors,188,189 macrobubbling aeration rates that optimize carbon utilization efficiency and productivity cannot meet culture demand.117,177,178The recent development of microbubbling technologies (10–50 μm diameter) enable greater mass transfer coefficients and longer residence times, which provide dissolved CO2 saturation without the need for elevated gas-phase concentration, or, continuous aeration with superior carbon utilization efficiency.185,190–193 Notably, bubble retention is maximized in tall reactors compatible with the thin format for efficient light utilization in flat panel and stacked waveguide reactors.55,120 Microbubble generation methods include fluidic oscillation applied to microporous diffusers (Fig. 9a)190,194 and break-through pressures applied to microporous membranes.195–197 A few potential disadvantages of microbubbling have been identified: (i) bursting in the culture provides shear forces which can kill cells;185 (ii) light scattering from bubble interfaces can reduce light penetration;185,198 and (iii) generation may require large energy use compared to macrobubble aeration. Further quantification is required for a fair economic comparison of these technologies.199 Aeration is also required to strip oxygen waste from the culture to concentrations recommended below 1 mM, or at most four times the atmospheric equilibrium concentration in water.200 Especially susceptible to toxic oxygen buildup, enclosed tube reactors require aeration nodes every ∼80 m of tube length primarily to manage oxygen holdup rather than replenish dissolved CO2.201–203
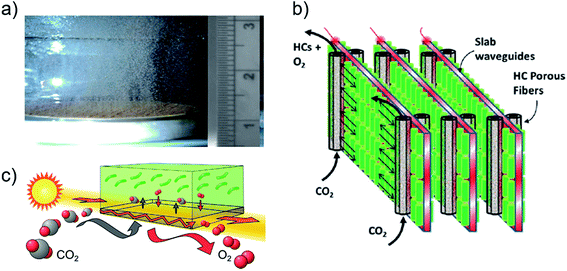 | ||
| Fig. 9 Carbon supply technologies in photobioreactors. (a) Microbubbles 20–100 μm diameter generated by fluidic oscillation (cm scale).190 (b) Schematic of reactor with hollow fiber membrane contactors for carbon supply interspacing planar waveguides for internal light supply.212 (c) Conceptual illustration of transparent, gas-permeable waveguide implemented in ref. 216. Images (a–c) are reproduced with permission from Elsevier. | ||
An alternative to bubbling aeration, the direct supply of dissolved CO2 to a culture combined with oxygen stripping may be provided by microporous membrane contactors, which have been widely used for microalgae-based wastewater treatment.183,201,204,205 Membrane contactors do not necessarily require operational energy for elevated pressure or flow,205,206 but mass transfer coefficients are generally lower than for microbubbling (and greater than for macrobubbling).175,195,198,205,207–210
A major disadvantage of current membrane contactor materials is opaqueness that blocks internal light transmission, which reduces productivity and limits reactor scalability, as exhibited in the series of studies that showed reduced productivity in stacked-waveguide reactors (mentioned above) when opaque hollow fiber membranes were added to improve carbon availability (Fig. 9b).211–213 The use of transparent nanoporous materials provide a compromise between internal light transmission and mass transfer barriers to CO2 supply and can be doubly used as internal waveguides in scalable reactor designs (Fig. 9c).206,214–216
3.4 Biofilm photobioreactors
The novel light and carbon management technologies cultivation reviewed here have proven laboratory-scale production advantages, but their potential to enable cost-effective production upon scale-up in pilot plant reactors has not been evaluated. Furthermore, novel reactor designs that undercut conventional pilot plant production costs by >50% may be required for economic biofuel production, according to some assessment studies.107,113,114,217,218 Being more complex than conventional approaches, these novel technologies may be economically feasible only for high-value product generation using suspension cultures. Irrespective of light and carbon management technologies, however, the management of large, commercial-production suspension culture volumes significantly contributes to production costs by each of the major factors outlined above – namely, infrastructure (by liquid volume), product extraction (by biomass harvesting and de-watering), operational energy and carbon supply (by flow and aeration pumping), and labor (by facility size and complexity related to each factor).11,55,112,116,219–223 Microalgal biofilms feature 2–3 orders of magnitude reduced liquid volumes220,224 and investigations of their potential to reduce water-related production costs of low-value biomass began in ∼2013.222,225,226Previously, microalgal biofilm reactors were developed mainly for municipal wastewater treatment, and later for coupled biomass and solely-biomass production.227–231 In these reactors, biofilms self-establish by natural recruitment and colonization, for which initial attachment is sensitive to and species-dependent on substrate properties (e.g., hydrophobicity, surface roughness and energy components) and materials, including waveguide substrates that enable illumination from both sides.140,232–234 Lipid content and production in biofilm reactors coupled with wastewater treatment can achieve comparable lipid content and biomass productivity as suspension cultures.235–239 Biofilms are submerged in media, optimally in a thin ∼mm layer using vertical drip irrigation, or intermittent contact with atmosphere and media on moving platforms (Fig. 10a). The thin liquid layer enhances gas transfer222,225,235,237,238,240 but the still-present gas–liquid boundary resistance (KLa) necessitated media aeration by bubbling for cultivation in some lab-scale studies.237,241 However, a year-long study of rotating biofilm reactors without active aeration or elevated CO2 supply demonstrated greater productivities compared to conventional reactors based on suspension culture ground areas.242
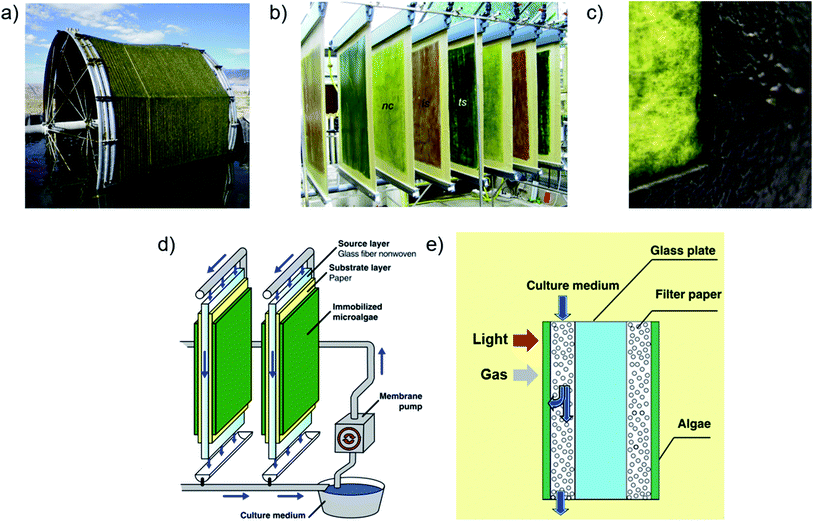 | ||
| Fig. 10 Biofilm-based photobioreactors. (a) Intermittently submerged rotating biofilm photobioreactor using cotton cord as a substrate.239 (b) “Twin-layer” biofilm reactors cultivating different species.225 (c) Photograph of a partly harvested biofilm of Halochlorella rubescens with ∼160 gDW m−2 grown on printing paper in a porous substrate photobioreactor.246 (d and e) Layout and microscale conceptual illustration of porous substrate photobioreactors.225,257 Images (a and c) are reproduced with permission from Elsevier. Images (b and d) are reproduced with permission from Springer. Image (e) is reproduced under the Creative Commons Attribution License [http://https://creativecommons.org/licenses/by/4.0/]. | ||
Porous substrate photobioreactors (PSBRs), developed in the last few years for biomass generation, isolate biofilms from direct media contact between porous substrates and gaseous surface exposure (Fig. 10b–e), which minimizes both KLa and the chance of spreading contaminants by liquid flow.223,225,226,243,244 By forcing cell attachment onto substrates – typically printing paper – stable biofilm cultivation of >80% of at least 90 strains have been demonstrated223,226 using this “attached cultivation” (previously “Twin-layer”) system. These species include those relevant to biofuel production, and at least one species sensitive to hydrodynamic shear forces161 that are absent in PSBRs.245 Other investigated substrates include solid and fibrous natural and synthetic polymers.189,228,238 Dissolved micronutrients are supplied to biofilms by perfusion though substrates from gravitational media flow from a pumped reservoir. Reactors are typically vertical-stack, as for flat panels, with light capture and cultivation occurring on both sides of the vertical flow reservoir. Micronutrient diffusion is mainly driven by concentration gradients from cell consumption,189,246,247 since the evaporation-driven component alone was shown insufficient for cultivation.223,248 Owing to the greater surface area, evaporative water loss from PSBRs compared to conventional (open pond) reactors are greater by ground area, but less per unit of biomass produced.249–251 PSBRs have consistently demonstrated superior productivity and light utilization efficiencies compared to conventional reactor cultivation. Most PSBR studies evaluate isolated biomass production, but some consider high-value product generation – for example, astaxanthin3,4 and to a lesser extent animal189 and aquaculture feedstock225 – and coupled operations with wastewater remediation;235,252 see Hoh et al. (2016) for a review of these coupled systems.228
Table 1 shows the cultivation conditions in different PSBRs that resulted in biomass productivities (linear growth regime) and maximum standing-crop densities achieved over the reported growth durations, based on cultivation surface area. Typically, PSBR productivities of 5–16 gDW m−2 d−1 are sustained for about a dozen days, but as high as 31 gDW m−2 d−1 was achieved for three days using Halochlorella rubescens.189,220,246,253,254 With 2–20 cm gaps between single PSBR layers that dilute solar intensities by ∼10× (comparable to flat-panel facility layouts),220,222,246,253 facility footprint productivities are typically 50–80 gDW m−2 d−1, as demonstrated using Scenedesmus obliquus and Halochlorella rubescens. These footprint productivities are several times that of the same species in conventional photobioreactors.189,222,253 The highest facility footprint productivities of conventional reactors is ∼40 gDW m−2 d−1.11,255,256 PSBRs have also achieved extremely high standing-crop densities of ∼200 gDW m−2 for Halochlorella rubescens and Haematococcus pluvialis, and 60 gDW m−2 for Botryococcus braunii.4,246,253 By footprint area, the standing crop density of Botryococcus braunii247,253 can be as high as 560 gDW m−2.
| Species | Biomass productivity† and standing crop density†† | Growth period (days) | Light cycle (hours per day) | PPFD (μmol m−2 s−1) | CO2(g)% v/v | Inoculation density (gDW m−2) | Ref. | |
|---|---|---|---|---|---|---|---|---|
| Spirulina platensis | gDW m−2 d−1† | 8–12 | 3 | 24 | 100–200 | 0.5 | 7–11 | 189 |
| gDW m−2 †† | — | — | — | — | — | — | ||
| Haematococcus pluvialis CCAC 0125 | gDW m−2 d−1 | 12–16 | 10–16 | 14 | 400–500 | 5 | 5 | 4 |
| gDW m−2 | 200 | 16 | 14 | 400–1015 | 5 | 5 | ||
| Halochlorella rubescens | gDW m−2 d−1 | 30 | 3 | 24 | 1023–1486 | 2–3 | 2–5 | 246 |
| gDW m−2 | 205 | 31 | 14 | 1011 | ∼0.04 | 2–5 | ||
| Botryococcus braunii SAG 807–1 | gDW m−2 d−1 | 6.5 | 8 | 24 | 100 | 1 | — | 247 |
| gDW m−2 | 60 | 8 | 24 | 100 | 1 | — | ||
| Botryococcus braunii FACHB 357 | gDW m−2 d−1 | 6.5, 5.5 | 2, 10 | 24 | 100 | 1 | — | 253 |
| gDW m−2 | 62 | 10 | 24 | 100 | 1 | — | ||
| Pseudochlorococcum sp. | gDW m−2 d−1 | 6–8 | 6 | 24 | 100 | 1 | 3–5 | 254 |
| gDW m−2 | ∼40 | 6 | 24 | 100 | 1 | 3–5 | ||
| Scenedesmus dimorphus | gDW m−2 d−1 | — | — | — | — | — | — | 257 |
| gDW m−2 | 107 | 10 | 24 | 100 | 1.5 | 8 | ||
| Aucutodesmus obliquus | gDW m−2 d−1 | ∼8 | 7 | 24 | 100 | 2 | 10 | 258 |
| gDW m−2 | — | — | — | — | — | — | ||
| Scenedesmus obliquus | gDW m−2 d−1 | 110 (70.9) | 3 (9) | 24 | 135 | 2 | 10 | 222 |
| gDW m−2 | 83.7 (450) | 8 (9) | 24 | 100 (135) | 2 | 10.6 (200) | ||
Light utilization efficiencies in PSBRs are superior to that of conventional reactors at moderate-high continuous light intensities 100–500 μmol m−2 s−1 with atmospheric CO2 (ref. 222, 246, 253 and 254) and at greater intensities with elevated CO2 due to the shift from light- to carbon-limited regimes.224,246 For example, at 700 μmol m−2 s−1 and a dilution factor of 10×, Scenedesmus productivity and photosynthetic efficiency was ∼7× that reported of an open pond suspension culture under the same conditions.222,257 At moderate intensities ∼200 μmol m−2 s−1, the addition of minimally elevated CO2(g) concentration (e.g. 0.12–0.5%) improves carbon utilization efficiencies and enhances productivities, for example, by >25× with Halochlorella rubescens (Fig. 11a).189,224,246,247,254 Of the carbon supply technologies reviewed here, PSBR may enable the closest approach to achieving the ultimate goal of using free, dilute atmospheric CO2 to meet high-density culture demand.176
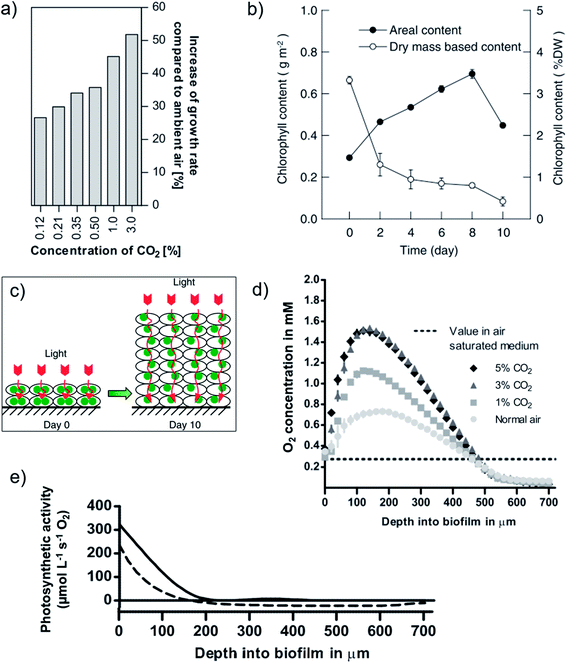 | ||
| Fig. 11 Growth characteristics of biofilms in porous substrate photobioreactors. (a) Halochlorella rubescens biofilm CO2-dependent increase of growth rate (%) compared to use of atmospheric CO2.246 (b) Reduction in per-cell chlorophyll content of Scenedesmus dimorphus with increasing biofilm growth due to photoacclimation ((c) schematic), which improves light distribution.257 (d and e) Depth profiles of Halochlorella rubescens biofilms exposed to 300 μmol m−2 s−1 and (e) aerated with 1% CO2(g) showing gross (solid line) and net (dashed line) photosynthetic activity.224 Image (a) is reproduced with permission from Elsevier. Images (b and c) are reproduced under the Creative Commons Attribution License [http://https://creativecommons.org/licenses/by/4.0/]. Images (d and e) are reproduced with permission from Wiley Periodicals. | ||
The recent development of a comprehensive kinetic model of PSBRs,259 combined with light and chemical microprofiling studies224,239,257 has provided great insight into light and carbon utilization in PSBRs. The fixed culture permits cell photoacclimation to local light intensities by pigment content expression, resulting in deeper light penetration (more light available per-cell)224,227,257 and tolerance to high light intensities that would inhibit production in suspension cultures (Fig. 11b and c).246 Also, density-dependent forward scattering in high-density biofilm cultures greatly enhances light dilution.223,248,257,259,260 With a greater photosynthetically active culture fraction than achievable in suspension cultures, PSBRs achieve greater biomass productivity and light utilization efficiency than conventional reactors, especially under high-light conditions.246,257 In addition, the minimal liquid layer on biofilms reduces reflection losses compared to submerged biofilm and suspension cultures and optimizes KLa.222,223 The latter enables high rates of CO2 uptake and O2 removal and results in the greatest net photosynthetic productivity at the biofilm surface, rather than deeper down – as is the case for submerged biofilms.224 The high-density production of dissolved O2 occurs throughout the photoactive culture fraction, however, which leads to supersaturated concentrations in the bulk that drives respiration (negative production) in cells below the light compensation point (Fig. 11d and e).224 From this point, increased biofilm thickness and respiratory culture fraction is observed as a decline in productivity from the linear growth regime.225,227,246,253,257
Aside from growth parameters of light and carbon, PSBR production is optimized via the species-dependent inoculation density of substrates189,254,258 and a cultivation period that avoids respiration losses. The onset of respiration can occur before ten days of cultivation and has occurred at 31 days at the latest in studies to-date.222,224,225,232,239,246,253,257,259 Biomass may be harvested by simple scraping, and contains 75–80% water content, which is comparable to centrifuged biomass from suspension cultures that initially contain ∼99% water.128,223 Compared to conventional reactors, harvesting biomass in PSBRs is highly energetically efficient and does not require flocculation.7,250 In summary, compared to conventional reactors, PSBRs exhibit superior light, carbon and water utilization efficiencies, and biomass productivities by facility area that do not necessarily suffer upon modular scale-up of the 2-D culture. PSBRs are then are a promising technology for economically feasible production of low-value biomass.
3.5 Alternative immobilized growth formats
The productivity and cost advantages of high-density and fixed culture cultivation enabled by biofilms and exploited in PSBRs can be alternatively achieved using matrix-immobilized microalgae. Immobilization matrices are widely used in wastewater remediation applications for the isolation of the culture from the environment and the ease of biomass reclamation.261–266 Alginate gels are popular immobilization matrices due to their relatively low cost and gentle encapsulation.264 We recently demonstrated high-density biomass production in a periodically harvested air-exposed alginate-immobilized microalgal culture, with comparable light utilization efficiencies and estimated material costs as reported of PSBRs223,246,267 (Fig. 12a). The different physiological state of matrix-immobilized compared to free microalgae can benefit production, depending on the species, density, and immobilization matrix; benefits include greater pigment concentration and lipid variety,265,268,269 lower senescence,270 comparable biomass productivities and light use efficiencies.262,264,271,272 Mutual symbiosis between multiple immobilized species can also benefit production, as recently reviewed in Santos and Reis (2014).263 Spatially organized co-cultivation (Fig. 12b) and synthetic mutualism with alginate-immobilized microalgae were recently separately demonstrated in proof-of-concept studies.273–275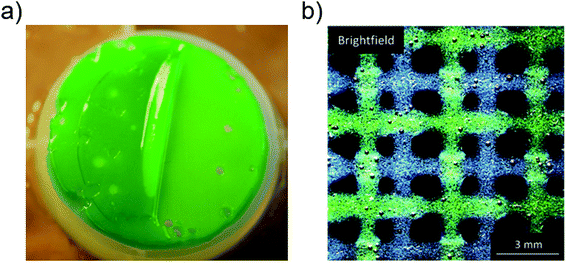 | ||
| Fig. 12 Alginate-immobilized cultivation of (a) partially harvested, air-exposed cyanobacteria culture267 and (b) printed scaffold with human bone cells and microalgae (green) species.275 Image (a) is reproduced with permission from WILEY-VCH Verlag GmbH. Image (b) is reproduced with permission from Wiley Periodicals. | ||
4 Optimizing downstream processing
Unlike terrestrial plants, microalgae are harvested as wet biomass which requires different downstream processing from crop-based biomass. To complete the microalgae-to-biofuel conversion route, pretreatment of biomass such as harvesting, thickening and dehydration are typically applied to simplify conversion processes.7,255,276–278 However, given the high latent heat vaporization for water (2265 kJ kg−1) the energy intensity and associated cost of drying is a fundamental challenge for microalgal biofuels. Given the fact that most microalgae have a total energy content on the order of 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 kJ kg−1,279,280 drying a microalgae slurry at a concentration below ∼20 wt% (which contains ∼3600 kJ kg−1 of energy) is of limited practicality for biofuel application. As such, the strategy to operate a microalgae operation is to either avoid massive drying or produce high-value products to offset the high processing costs. In this section, we will first discuss the dewatering and drying processes, then describe how dry biomass, wet biomass, microalgae in culture and direct secretion can be implemented. Microalgae in culture involve directly using cells, wet biomass is typically concentrated from harvested cells and dry biomass is obtained by drying wet biomass. Each area will be briefly reviewed while highlighting emerging technologies (Table 2).
000 kJ kg−1,279,280 drying a microalgae slurry at a concentration below ∼20 wt% (which contains ∼3600 kJ kg−1 of energy) is of limited practicality for biofuel application. As such, the strategy to operate a microalgae operation is to either avoid massive drying or produce high-value products to offset the high processing costs. In this section, we will first discuss the dewatering and drying processes, then describe how dry biomass, wet biomass, microalgae in culture and direct secretion can be implemented. Microalgae in culture involve directly using cells, wet biomass is typically concentrated from harvested cells and dry biomass is obtained by drying wet biomass. Each area will be briefly reviewed while highlighting emerging technologies (Table 2).
| Feedstock type | Conversion/extraction techniques | Main products | Reference |
|---|---|---|---|
| Dry powder | Mechanical disruption and chemical extraction | Antioxidants, pigments, PUFA, additives | 151 and 319 |
| Direct combustion | Heat | 320 | |
| Pyrolysis | Bio-oil | 321 | |
| Microalgae slurry (>15% TSS) | Hydrothermal liquefaction | Biocrude | 322–325 |
| Supercritical water gasification | Syngas | 326–328 | |
| Microalgae in culture (<10% TSS) | Fermentation | Bioethanol | 329–335 |
| Anaerobic digestion | Methane | 336–340 | |
| Direct secretion | Hydrogen, alcohols or alkanes | 341–343 | |
| Microbial fuel cells | Electricity | 344–352 |
4.1 Harvesting and dewatering processes
Initial harvesting is typically accomplished by either screening or sedimentation, where the concentration is increased from 0.1% of total suspended solids (TSS) to a slurry of about 2–7% TSS.7,11,278,281 In terms of screening, microstraineres and vibrating screens are the most commonly used. Standalone sedimentation by gravity is highly energy efficient for large cells281,282 but is unsuitable for many types of microalgae. Sedimentation can be enhanced by forming larger aggregates of cells, typically by chemical flocculation and coagulation. Ultrasound-assisted harvesting forces cell aggregation at acoustic nodes.283 This technique is free of chemical additives, operated continuously and has the flexibility of adopting different types and sizes of cells by adjusting the operating parameters such as acoustic energy density, contrast factor, and ultrasound frequency. Lab-scale experiments have demonstrated a concentration factor of 11.6 can be achieved at a flow rate of 25 mL min−1.283 Other initial harvesting techniques includes air flotation,284,285 and electric field assisted harvesting.7,278 As discussed, biofilm cultivation143,286 has unique harvesting advantages but further development is required to make them cost and energy effective for biofuel production.After initial harvesting, thickening techniques can be applied to concentrate the algae suspension to above 15% of TSS.7,278,281 Two major thickening techniques are centrifugation and filtration. Thickening saves a significant amount of energy compared to directly drying the harvested biomass10,287,288 but requires extra capital investment. The energy cost for this process depends on the characteristics of the cells, system design, and desired output concentration. Moreover, as the desired output concentration increases, the energy cost associated with incremental percentage of dry biomass climb steeply.279,288 Centrifugation is commonly used in the lab and provides rapid water removal but it is very energy intensive, thus not practical at industrial scale for bioenergy applications, but is widely used to produce high-value products such as pigments, polyunsaturated fatty acids (PUFAs), phycobiliproteins, enzymes and toxins.151,289–291 Filtration is conceptually simple but potentially difficult to operate due to the two major issues: (1) the pore size needs to be small to increase efficiency but not too small to cause clogging issues and reduce flow rates; (2) easy recovery of algal biomass from the filter is desired without using the backwash which will lead to re-dilution of the product.236,278,279
Dehydration is used to achieve the final high level of biomass concentration so that conventional extraction methods and infrastructure used for terrestrial plants can be used effectively. Typical dehydration techniques include solar drying, spray drying, freeze drying and belt drying. Solar drying is relatively easy to implement but it is hindered by the lack of control (overheating, weather dependency), long drying time and possible biomass degradation.11,292–295 Other drying processes are far too costly and energy intensive to be applicable for bioenergy applications.
4.2 Processing for dry microalgae
Once microalgae biomass is completely dried through dehydration techniques, conventional physical disruption followed by chemical extraction and conversion techniques can be directly applied. Effective physical disruption can offset the need for harsh processing conditions required in the process of chemical extraction.276,290,295 Cell homogenization and bead milling are commonly used in the industry, and emerging disruption techniques such as microwave, pulsed electric field and ultrasonic are still under development.282,295,296 Among these techniques, microwave-assisted extraction (MAE) has attracted lots of attention due to its advantage of easy operation, high energy transfer efficiency, rapid heating and relatively low cost. MAE has been investigated to be highly effective for pigments297 and lipids298 extraction from microalgae. MAE of fucoxanthin from Cylindrotheca closterium for 5 min enabled maximal extraction equivalent to 60 min of conventional solvent extraction method.297 With the addition of ionic liquids, MAE has been applied to extract lipids from wet microalgal biomass where extraction rates were increased by an order of magnitude in most cases.299 However, the cost associated with these disruption techniques is still too high for biofuel production. The requirements for physical disruption depend on the characteristics of the microalgae and specific extraction techniques could be simplified, or even eliminated, by genetic engineering or extraction process enhancement.The most commonly used lipid extraction technique for dry microalgal biomass is organic solvent extraction which is found to be highly effective and cost efficient.282,296 Supercritical CO2 extraction is not suitable for biofuel production due to its high initial and maintenance cost, but its application has increased in the nutraceutical and biochemical industry in recent years.300–303 The major advantage of using supercritical CO2 as solvent is to produce contamination-free products and the associated cost can be offset by the high marginal value bioproducts. Other extraction techniques such as accelerated solvent, two-phase solvents and switchable-solvent extractions have been proposed and demonstrated to improve the extraction efficiency of intracellular components from microalgae.304–306
Lipids extracted from microalgae can be further converted to biodiesel through several pathways: chemical transesterification, enzymatic conversion and catalytic upgrading. Among these, chemical transesterification is relatively mature and has been used widely for biodiesel production with conversion efficiencies above 90%.307–309 However, algal oils are typically highly complex in contrast with vegetable oils, containing fatty acids, phospholipids, carotenoids, chlorophyll and other components in various composition.279,310 Therefore, in order to accelerate this reaction, obtain higher conversion rate and reach the full potential of commercialization, a full understanding of strain specific oil composition is required. In contrast, enzymatic conversion uses biological catalysts (lipases) instead of acids or bases to convert lipids into biodiesel with less processing energy, easier recycling of catalysts and less alkaline wastewater pollution. Although, enzymatic approaches have advantages over conventional methods, other challenges associated with the cost of lipase, operational life, tolerance of the environment for enzymes and harvesting strategies for products need to be addressed for this path to run at a commercial scale.311–313 Catalytic upgrading of algal lipids into renewable gasoline, jet fuel and diesel can also be achieved by hydrotreating processes which react oils with hydrogen at high temperature and pressure in the presence of catalysts.314–316 This conversion process is commonly used in the petroleum industry to upgrade crude oil to produce a wide multitude of performance specified fuels. Ideally, this process leverages existing techniques and infrastructure albeit with catalysts and process parameters tuned for algal feedstocks.
Recent research in algal downstream processing, has shifted from a lipid-centric approach to a more holistic approach. If one can obtain value from all feedstock components the commercialization of microalgae technology will be more economic viable. For example, residual carbohydrates and proteins from microalgae can be used to enrich the nutrition in animal feed or further processed to produce biofuels through other conversions such as fermentation, anaerobic digestion and hydrothermal liquefaction. Another approach to achieve commercially feasibility in microalgae industry is to produce high-value bioproducts.13,39,55,317,318 These bioactive components present a great interest in pharmaceutical, cosmetic and nutraceutical industries and can easily offset the high costs associated with biomass processing. The understanding of microalgae and development of technology gained in this approach will accelerate the accomplishment of producing sustainable energy from microalgae.
Without extracting lipids, direct combustion and pyrolysis have been investigated to directly produce energy from whole algae. Direct combustion of dry microalgae biomass releases the largest amount of energy. However, multiple life cycle assessments10,353,354 indicate drying the microalgae into powders costs more energy than the biomass contained, plus the challenges associated with ash content and emission control make direct combustion of microalgae impractical on a commercial scale.355–357 Moreover, the energy released from direct combustion is in the form of heat which is less desirable than liquid biofuels used for transportation. Pyrolysis is a thermal decomposition process in absence of oxygen at high temperature (300–1000 °C), converting organic components into a wide range of products with hydrocarbon rich liquid (bio-oil) as the desired product.358–360 Recent research321 indicated that fast or flash pyrolysis of microalgae with a reaction time of 2–3 s, reaction temperature around 500 °C with a heating rate of 600 °C s−1 is capable of achieving 18–24% liquid yields with higher heating value of 29 MJ kg−1. Pyrolysis bio-oil from microalgae has lower oxygen content and viscosity and larger higher heating value than that from woody biomass but still requires extensive refining for use in conventional fuel engines. More importantly, similar to direct combustion, the major roadblock in pyrolysis is removing the moisture content in the biomass which cause the overall process to be energy negative.
4.3 Processing for wet microalgae biomass
Avoiding the energy and cost intensive thickening and drying processes dramatically alleviates the energy burden for microalgae biofuel applications. Two promising conversion processes applicable to wet biomass at a concentration above 15% TSS are hydrothermal liquefaction (HTL) and supercritical water gasification (SCWG).HTL is one of the most promising conversion pathway for biofuel production due to the ability to employ raw wet feedstock, a fast reaction rate, a high-energy return on investment, good characteristics of biocrude, a low production of char, and the potential for recycling of nutrients (Fig. 13a and b). Similar to natural formation of petroleum-based fossil fuels, HTL converts biomass into biocrude at high temperature (200–380 °C) and pressure (5–28 MPa) in the presence of water, albeit on a timescale of minutes by chemically and physically cracking down large biomolecules into small fractions.322,324,325,361,362 Compared to other thermochemical conversion techniques such as pyrolysis, the higher heating value of HTL biocrude is about 35 MJ kg−1, much higher than typical pyrolysis bio-oil with a value of 20–25 MJ kg−1.363
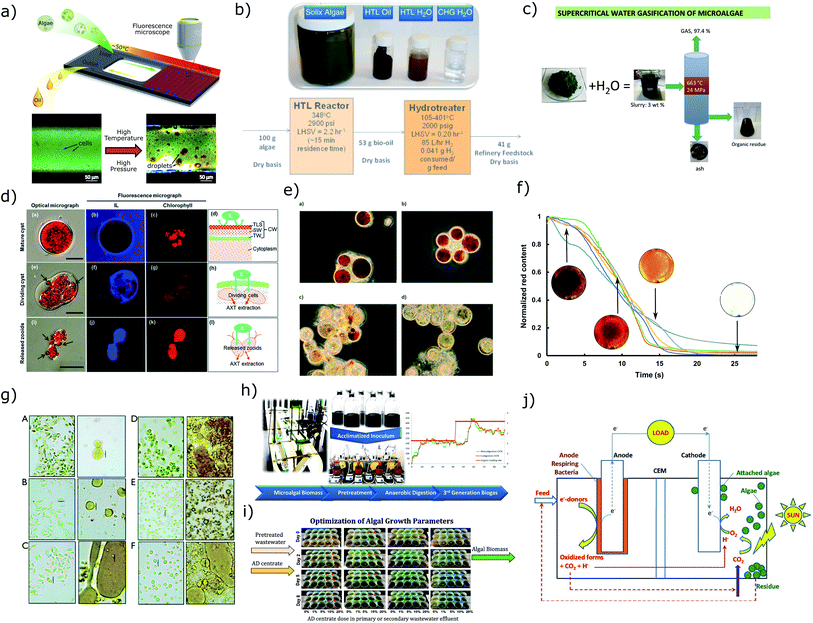 | ||
| Fig. 13 Summary of current downstream processing techniques for wet microalgae biomass (a–f) and cells in culture (g–j). (a–b) Hydrothermal liquefaction.322,323 Image (b) has been reproduced with permission from Elsevier,323 Copyright 2013. (c) Supercritical water gasification.327 Reproduced with permission from Elsevier, Copyright 2016. (d–e) Ionic liquid treatment for wet extraction.381,382 Image (d) has been reproduced with permission from Royal Society of Chemistry,381 Copyright 2014. Image (e) has been reproduced with permission from Royal Society of Chemistry,382 Copyright 2015. (f) Astaxanthin extraction for hydrothermal disrupted cells.380 (g) Fermentation of pretreated wet biomass.329 Image reproduced under CC-BY license, Copyright 2014. (h–i) Anaerobic digestion of algal biomass.337,338 Image (h) has been reproduced with permission from Elsevier,338 Copyright 2017. Image (i) has been reproduced with permission from Elsevier,337 Copyright 2016. (j) Microalgae-microbial fuel cells.402 Reproduced with permission from Elsevier, Copyright 2015. | ||
A fundamental challenge in HTL of biomass is optimizing the processing conditions (temperature, pressure, residence time and heating rate) to obtain optimal yield and efficiency.361,364,365 To this end, HTL324,366,367 with a fast heating rate was investigated and resulted in higher biocrude productivity. However, the heating rate in this experiment was limited at 230 °C min−1 which was not able to explore the peak productivity with respect to heating rate. By eliminating the limits from conventional batch reactors, microdevices322 that allow direct observation of HTL (Fig. 13a) have been developed to precisely control processing parameters and perform real-time monitoring. The results indicate the higher heating values of biocrude approached saturation within 1 min due to early mechanical disruption of cells that enabled solvent extraction. Therefore, the HHV alone does not present the quality of biocrude whereas chemical composition and physical characteristics should also be considered.
A sequential HTL process368 was also performed to maximize the biocrude productivity and minimize the bio-char formation. A low temperature (140–200 °C) HTL was used as the first step to disrupt the cell and release intracellular products, polysaccharides in this case. The second step utilize a higher temperature (220–300 °C) HTL that converts the remaining biomass to biocrude which resulted in overall higher biocrude productivity and less bio-char. Lower polysaccharide content resulting in decreased bio-char production also agrees with HTL of individual categories of biomass feedstocks which indicates polysaccharides generates more solid products than protein and lipids.364 Similar to HTL, hydrothermal carbonization (HTC) uses reaction temperatures in the lower range (180–250 °C) with slightly elevated pressure (2–10 MPa) which tends to produce more solid products instead of liquid products.369–371 A recent comparison of torrefaction and HTC of lignocellulosic biomass indicates that HTC as the wet torrefaction method is the more favorable process in terms of energy content, hydrophobicity, and inorganic components of the solid products.372
Analogous to HTL, SCWG reacts at higher temperature (400–700 °C) and pressure (>22 MPa) to decompose biomolecules to produce syngas containing hydrogen, carbon monoxide and methane with a small quantity of solid and liquid products326–328,373 (Fig. 13c). Gasification is commonly combined with Fischer–Tropsch Synthesis (FTS) to convert syngas into liquid fuels.374–376 The major advantage of this pathway is the flexibility to produce a wide variety of fuels and products with known properties. Recent research indicates the issues for developing this process are: precipitation of inorganic salts, an unclear reaction mechanism associated with reaction temperature, pressure, heating rate and wall effects, the requirement of effective catalyst and high-temperature-resistant materials, and high energy costs.359,371,373 Conventional gasification (in an environment of insufficient oxidizer) of microalgae in a temperature range of 800–1000 °C was also studied and due to the requirement of dry biomass, this pathway for biofuel production usually resulted in negative net energy.11,357,377
Another interesting application of subcritical water is to lyse cells for extraction of high-value intracellular products.378–380 A particular challenge is breaking robust cell walls to allow extraction, particularity with wet biomass.380 Lab scale tests have shown that hydrothermal (Fig. 13f) and ionic liquids (ILs) (Fig. 13d and e) can achieve comparable efficiencies as mechanical processing to dry biomass. Hydrothermal disruption of the cell wall at 200 °C effectively enabled solvent extraction of astaxanthin to achieve more than 95% efficiency as opposed to control with a 7.5% in extraction efficiency.380 ILs have been used as extracting agents to replace organic solvents or pre-treatment material to disrupt the cell wall to enhance extraction. An extraction efficiency of 82% was achieved using 1-ethyl-3-methylimidazolium ethylsulfate, but a germination process of H. pluvialis cysts is required to weaken the cell wall.381 ILs are also applied in a pre-treatment process to achieve >70% extraction efficiency, but the experiment utilized dry cell powders as feedstock which normally has weaker cell walls and the reusability of ILs needs improvement for large scale production.382
There are also other approaches to directly extract intracellular lipids from wet biomass,277,383–385 but due to either high cost or low extraction efficiency none of them have been commercialized yet. For biofuel productions, since the current wholesale price for diesel is down to about 70 cents per liter, bringing additional steps or expensive additives into the process is most likely a non-starter.
4.4 Processing for microalgae in culture
To minimize the energy used in removing water from biomass, biologic conversion processes involving fermentation and anaerobic digestion have been studied. In these pathways, biofuels can be either produced directly from microalgae culture or after initial harvesting with minimal energy cost. Lastly this section includes microalgae–microbial fuel cell approaches whereby microalgae are employed for direct electricity production.Fermentation is a well-established method used in alcohol production to convert carbohydrates into ethanol by yeast. There are two major approaches to produce ethanol from microalgae: (1) like yeast, some microalgae such as Chlorella and Chlamydomonas can produce alcohols through heterotrophic fermentation;330,331,386,387 (2) microalgae containing a significant amount of carbohydrates can be used as a sugar source for yeast fermentation.332,333,335 In the first approach, sugars can either be generated internally from the synthesis of microalgae or fed to algae externally, however the process lacks the productivity of yeast fermentation. The second approach usually requires pretreatment to promote hydrolysis of the cell wall to: (1) access to intracellular components; and (2) release fermentable sugars. Although research334 indicated the sugar released from wet microalgal biomass was lower than that from dried biomass, drying the biomass prior to conversion does not provide an energy return on investment.353,354,357 Recent research329 (Fig. 13g) demonstrated that acid-catalyzed pretreatment prior to lipids extraction released more than 90% of fermentable sugars for wet microalgae biomass. Although noticeable achievements have been made in this pathway, significant breakthroughs are required to produce biofuels economically at industry scale with this approach.
Anaerobic digestion used in wastewater treatment to produce methane has the potential to exploit the entire organic carbon content of microalgae without the requirement for drying (Fig. 13h and i). It can also significantly benefit from the direct use of existing infrastructure and experience in the field of wastewater treatment.336–338,388,389 Methane as the main product in the process not only can be used as fuel but can also be converted to bioplastics to increase the value.339,340,390 However, this pathway is hindered by the low practical methane yields mainly due to the resistance of microalgal cell walls. Rigid cell walls of microalgae not only reduce the amount of digestible substrate but also limit the access of microorganisms to intracellular components resulting in low reaction rate.388,389,391,392 Thermal, chemical and mechanical pretreatments of microalgae were used to improve methane yields but the energy cost involved in the extra steps is higher than the energy gain from increased methane production.336,389,393 By tuning the pH and retention time, providing heat treatment and addition of methanogen inhibitors, this process can be altered to produce hydrogen387,394 instead of methane but it requires significant development to be commercially available. The more likely role for anaerobic digestion is in combination with other methods to fully harvest the remaining value of biomass and therefore maximize the biofuel production.
4.5 Direct secretion from living cells
Direct secretion of products such as hydrogen,395,396 alcohols342,397,398 and alkanes341,343,399 from microalgae culture can be achieved through genetic engineering. Hydrogen production from Scenedesmus obliquus was first observed by Gaffron and Rubin400 and having an anaerobic environment was later found to be critical for this process.401 Sulfur deprivation was used to inhibit the activity of PSII and enhance the activity of hydrogenase enzymes for hydrogen production. Breakthroughs in genetic engineering, processing parameter control on sulfur quantity and immobilization of cells are expected to increase hydrogen productivity by further inhibiting PSII activity and increasing the activity of hydrogenase enzymes. Secreted hydrogen can be easily collected, however the low conversion efficiency remains a major roadblock for this pathway. Direct secretion of alcohols and alkanes appears to be a promising alternative but most information in this area is proprietary. Secretion of triterpene from Botryococcus braunii was reported to reach a volumetric productivity of 22.5 mg per L per photo-h at a high cell concentration of 20 gDW L−1.283 These pathways usually require cheap source of CO2 or sugars as feedstock and development to boost the feedstock-to-product conversion rates.Microalgae–microbial fuel cells biologically convert solar energy to electrical energy and while the technology is maturing, the development of this conversion strategy is at an early stage.345,346,348,349,402 Microbes at the anode generate electrons, protons and CO2 by digestion of organics and the microalgae at the cathode takes CO2, light, proton and electrons to grow through photosynthesis (Fig. 13j). Also known as biological photovoltaics, or BPVs, this approach has seen many recent advances. Microfluidic approaches were applied to perform a qualitative investigation of several key factors including cell density, electron mediator concentration and light intensity and indicated the major obstacle for power production from BPVs is the transport of reducing equivalents across the cytoplasmic membrane.344,350 Understanding of electron transport mechanism is also believed to be essential for selection of photosynthetic microbes to enhance electrical output.347,352 Furthermore, the overall performance of BPVs is strongly associated with surface morphology and corresponding material characteristics,403 manipulation of thylakoid terminal oxidases404,405 and types of feedstocks.406 One of the challenges with this approach is the need for electrodes which tend to block light paths in a way similar to the CO2 delivery mechanisms discussed earlier. A proof-of-concept cell was developed whereby both light and electrons were delivered via a metallic/plasmonic surface,351 however, that approach is not well suited to large scale production for a number of reasons. In general, there are many challenges with microalgal–microbial fuel cell approaches including technical obstacles, high operation costs and low power output that need to be solved for this process to be economically feasible. Similar to some other approaches above, this strategy can be more readily adopted in a wastewater treatment context, where the primary objective is remediation and power production is a side benefit. We note however, that this research area is adapting quickly and may well produce surprises.407
5 Perspective
The potential for biofuel and bioproducts from microalgae to replace fossil fuels and convert CO2 into valuable products with low land use and without competing with food supply is, of course, very attractive. However, in practice, the limitations of production from microalgae currently constrain widespread adoption. This is especially true given the competitive technological landscape in sustainable energy with impressive developments in photocatalysis and electrocatalysis (coupled with inexpensive solar photovoltaics) side-stepping many of the limitations of photosynthesis – particularly the low conversion efficiency.408,409Nevertheless, microalgae remain the only sustainable and scalable option for the production of complex molecules such as nutraceuticals and long-chain hydrocarbons from solar energy. As such, overcoming the limitations of producing fuels and products from microalgae remains important. To this end, technology to inform and optimize microalgal cultivation and processing is rapidly developing.
As these technologies develop, removing current barriers is essential for application beyond a narrow niche. Multiplexed experimental platforms to discover optimal culture conditions provide a great advantage over conventional techniques in terms of throughput, they currently suffer from key limitations. First, they lack versatility in terms of spectral control. Second, the complexity of these devices limit their usability and consequently their industrial adoption. Finally, there is a great opportunity for improving the readouts possible with multiplexed platforms. The development of cultivation technology must not only maximize productivity – but must also produce in a manner conducive to efficient harvesting and downstream processing. Further, most of the technologies discussed in this review currently remain at lab-scale. While critical information can be obtained at the laboratory scale, production and processing technology will need to be scaled to make a widespread impact. Simplification of effective but complex lab-scale approaches will be essential for growth, and widespread adoption.
Acknowledgements
This work was supported through a Strategic Grant from the Natural Science and Engineering Research Council of Canada, the University of Toronto Connaught Global Scholars Program in Bio-Inspired Ideas for Sustainable Energy, the University of Toronto McLean Senior Fellowship (DS), E.W.R. Steacie Memorial Fellowship (DS), and the Canada Research Chairs Program. PG gratefully acknowledges NSERC PGS and Hatch Scholarships. BN gratefully acknowledges funding from Ontario Graduate Scholarships, the Queen Elizabeth II Graduate Scholarships in Science & Technology, NSERC CGS Scholarships and the MEET, NSERC CREATE Program. Ongoing infrastructure support from the Canadian Foundation for Innovation and operational support through the NSERC Discovery Program is also gratefully acknowledged, as is imaging support from the Centre for Microfluidic Systems.References
- M. D. Ooms, C. T. Dinh, E. H. Sargent and D. Sinton, Nat. Commun., 2016, 7, 12699 CrossRef CAS PubMed.
- D. Sinton, Lab Chip, 2014, 14, 3127–3134 RSC.
- M. Wan, D. Hou, Y. Li, J. Fan, J. Huang, S. Liang, W. Wang, R. Pan, J. Wang and S. Li, Bioresour. Technol., 2014, 163, 26–32 CrossRef CAS PubMed.
- A. C. Kiperstok, P. Sebestyén, B. Podola and M. Melkonian, Algal Res., 2017, 21, 213–222 CrossRef.
- S. a. Scott, M. P. Davey, J. S. Dennis, I. Horst, C. J. Howe, D. J. Lea-Smith and A. G. Smith, Curr. Opin. Biotechnol., 2010, 21, 277–286 CrossRef CAS PubMed.
- E. Stephens, I. L. Ross, J. H. Mussgnug, L. D. Wagner, M. a. Borowitzka, C. Posten, O. Kruse and B. Hankamer, Trends Plant Sci., 2010, 15, 554–564 CrossRef CAS PubMed.
- N. Uduman, Y. Qi, M. K. Danquah, G. M. Forde and A. Hoadley, J. Renewable Sustainable Energy, 2010, 2, 012701 CrossRef.
- N. Norsker, M. J. Barbosa, M. H. Vermuë and R. H. Wijffels, Biotechnol. Adv., 2011, 29, 24–27 CrossRef CAS PubMed.
- G. Luísa, SpringerBriefs in Microbiology, 2011 Search PubMed.
- L. Lardon, A. Hélias, B. Sialve, J.-P. Steyer and O. Bernard, Environ. Sci. Technol., 2009, 43, 6475–6481 CrossRef CAS PubMed.
- L. Brennan and P. Owende, Renewable Sustainable Energy Rev., 2010, 14, 557–577 CrossRef CAS.
- X. Zhou, S. Yuan, R. Chen and R. M. Ochieng, J. Renewable Sustainable Energy, 2015, 7, 012701 CrossRef.
- E. C. Odjadjare, T. Mutanda and A. O. Olaniran, Crit. Rev. Biotechnol., 2015, 8551, 1–16 Search PubMed.
- Y. T. Yang and C. Y. Wang, Micromachines, 2016, 7, 1–14 Search PubMed.
- V. N. Goral and P. K. Yuen, Ann. Biomed. Eng., 2012, 40, 1244–1254 CrossRef PubMed.
- X. Su, E. W. K. Young, H. a. S. Underkofler, T. J. Kamp, C. T. January and D. J. Beebe, J. Biomol. Screening, 2011, 16, 101–111 CrossRef CAS PubMed.
- E. K. Sackmann, A. L. Fulton and D. J. Beebe, Nature, 2014, 507, 181–189 CrossRef CAS PubMed.
- Y. J. Sung, J. Y. H. Kim, K. W. Bong and S. J. Sim, Analyst, 2016, 141, 989–998 RSC.
- B. Nguyen, P. J. Graham and D. Sinton, Lab Chip, 2016, 16, 2785–2790 RSC.
- P. J. Graham, J. Riordon and D. Sinton, Lab Chip, 2015, 15, 3116–3124 RSC.
- G. Yu, Y. Li, G. Shen, W. Wang, C. Lin, H. Wu and Z. Chen, J. Appl. Phycol., 2009, 21, 719–727 CrossRef CAS.
- M. Prussi, M. Buffi, D. Casini, D. Chiaramonti, F. Martelli, M. Carnevale, M. R. Tredici and L. Rodolfi, Biomass Bioenergy, 2014, 67, 390–400 CrossRef CAS.
- I. Perner-Nochta and C. Posten, J. Biotechnol., 2007, 131, 276–285 CrossRef CAS PubMed.
- A. K. Moberg, G. K. Ellem, G. J. Jameson and J. G. Herbertson, J. Appl. Phycol., 2012, 24, 357–363 CrossRef CAS.
- A. K. Gebremariam and Y. Zarmi, J. Appl. Phys., 2012, 111, 34904 CrossRef.
- J. Hardin, G. Bertoni and L. Kleinsmith, Becker's World of the Cell, Pearson, 8th edn, 2012 Search PubMed.
- J. U. Grobbelaar, Photosynth. Res., 2010, 106, 135–144 CrossRef CAS PubMed.
- A. P. Carvalho, S. O. Silva, J. M. Baptista and F. X. Malcata, Appl. Microbiol. Biotechnol., 2011, 89, 1275–1288 CrossRef CAS PubMed.
- M. Sarcina, N. Bouzovitis and C. W. Mullineaux, Plant Cell, 2006, 18, 457–464 CrossRef CAS PubMed.
- D. Kirilovsky, Photosynth. Res., 2007, 93, 7–16 CrossRef CAS PubMed.
- A. Losi and W. Gärtner, Photochem. Photobiol., 2011, 87, 491–510 CrossRef CAS PubMed.
- B. L. Montgomery, Mol. Microbiol., 2007, 64, 16–27 CrossRef CAS PubMed.
- A. K. Singh, M. Bhattacharyya-Pakrasi, T. Elvitigala, B. Ghosh, R. Aurora and H. B. Pakrasi, Plant Physiol., 2009, 151, 1596–1608 CrossRef CAS PubMed.
- P. Hartmann, Q. Béchet and O. Bernard, Bioprocess Biosyst. Eng., 2014, 37, 17–25 CrossRef CAS PubMed.
- H. Takache, G. Christophe, J. F. Cornet and J. Pruvost, Biotechnol. Prog., 2010, 26, 431–440 CAS.
- H. Takache, J. Pruvost and H. Marec, Algal Res., 2015, 8, 192–204 CrossRef.
- S. Xue, Z. Su and W. Cong, J. Biotechnol., 2011, 151, 271–277 CrossRef CAS PubMed.
- K. L. Terry, Biotechnol. Bioeng., 1986, 28, 988–995 CrossRef CAS PubMed.
- A. Solimeno, R. Samsó, E. Uggetti, B. Sialve, J.-P. Steyer, A. Gabarró and J. García, Algal Res., 2015, 12, 350–358 CrossRef.
- A. Bernardi, G. Perin, E. Sforza, F. Galvanin, T. Morosinotto and F. Bezzo, Ind. Eng. Chem. Res., 2014, 53, 6738–6749 CrossRef CAS PubMed.
- T. Hauser, L. Popilka, F. U. Hartl and M. Hayer-Hartl, Nat. Plants, 2015, 1, 15065 CrossRef CAS.
- F. Rubio Camacho, F. García Camacho, J. M. Fernández Sevilla, Y. Chisti and E. Molina Grima, Biotechnol. Bioeng., 2003, 81, 459–473 CrossRef CAS PubMed.
- S. Abu-Ghosh, D. Fixler, Z. Dubinsky and D. Iluz, Bioresour. Technol., 2016, 203, 357–363 CrossRef CAS PubMed.
- A. Han, H. Hou, L. Li, H. S. Kim and P. de Figueiredo, Trends Biotechnol., 2013, 31, 225–232 CrossRef CAS PubMed.
- Y. J. Juang and J. S. Chang, Biotechnol. J., 2016, 11, 327–335 CrossRef CAS PubMed.
- S. G. Hong, M. Song, S. Kim, D. Bang, T. Kang, I. Choi and L. P. Lee, ACS Nano, 2016, 10, 5635–5642 CrossRef CAS PubMed.
- M. Chen, T. Mertiri, T. Holland and A. S. Basu, Lab Chip, 2012, 3870–3874 RSC.
- H. S. Kim, T. L. Weiss, H. R. Thapa, T. P. Devarenne and A. Han, Lab Chip, 2014, 14, 1415–1425 RSC.
- X. Lou, G. Kim, H. K. Yoon, Y.-E. K. Lee, R. Kopelman and E. Yoon, Lab Chip, 2014, 14, 892–901 RSC.
- C. W. Mullineaux, M. J. Tobin and G. R. Jones, Nature, 1997, 390, 421–424 CrossRef CAS.
- K. Ozasa, J. Lee, S. Song, M. Hara and M. Maeda, Lab Chip, 2011, 11, 1933–1940 RSC.
- K. Ozasa, J. Lee, S. Song, M. Hara and M. Maeda, Appl. Soft Comput., 2013, 13, 527–538 CrossRef.
- B. J. Kim, L. V. Richter, N. Hatter, C.-K. Tung, B. a. Ahner and M. Wu, Lab Chip, 2015, 18–25 CAS.
- S. P. Forry and L. E. Locascio, Lab Chip, 2011, 11, 4041–4046 RSC.
- J. Ruiz, G. Olivieri, J. de Vree, R. Bosma, P. Willems, J. H. Reith, M. H. M. Eppink, D. M. M. Kleinegris, R. H. Wijffels and M. J. Barbosa, Energy Environ. Sci., 2016, 9, 3036–3043 Search PubMed.
- J. M. Clomburg, A. M. Crumbley and R. Gonzalez, Science, 2017, 355, aag0804 CrossRef PubMed.
- S. C. C. Shih, N. S. Mufti, M. D. Chamberlain, J. Kim and A. R. Wheeler, Energy Environ. Sci., 2014, 7, 2366–2375 CAS.
- C. W. Kim, M. Moon, W. K. Park, G. Yoo, Y. E. Choi and J. W. Yang, Biotechnol. Bioprocess Eng., 2014, 19, 150–158 CrossRef CAS.
- Y. Kim, S. N. Jeong, B. Kim, D. P. Kim and Y. K. Cho, Anal. Chem., 2015, 87, 7865–7871 CrossRef CAS PubMed.
- M. D. Ooms, P. J. Graham, B. Nguyen, E. H. Sargent and D. Sinton, Biotechnol. Bioeng., 2017, 9999, 1–10 Search PubMed.
- N. Yoshimoto, T. Sato and Y. Kondo, J. Appl. Phycol., 2005, 17, 207–214 CrossRef CAS.
- C. Brindley, N. Jiménez-Ruíz, F. G. Acién and J. M. Fernández-Sevilla, Algal Res., 2016, 16, 399–408 CrossRef.
- R. Rosello Sastre, Z. Csögör, I. Perner-Nochta, P. Fleck-Schneider and C. Posten, J. Biotechnol., 2007, 132, 127–133 CrossRef CAS PubMed.
- D. Zhang, P. Dechatiwongse and K. Hellgardt, Algal Res., 2015, 8, 99–107 CrossRef.
- P. H. C. Eilers and J. C. H. Peeters, Ecol. Modell., 1988, 42, 199–215 CrossRef.
- P. H. C. Eilers and J. C. H. Peeters, Ecol. Modell., 1993, 69, 113–133 CrossRef CAS.
- B.-P. Han, J. Theor. Biol., 2002, 214, 519–527 CrossRef CAS PubMed.
- A. Richmond, Z. Cheng-Wu and Y. Zarmi, Biomol. Eng., 2003, 20, 229–236 CrossRef CAS PubMed.
- Y. Zarmi, G. Bel and C. Aflalo, in Handbook of Microalgal Culture: Applied Phycology and Biotechnology, 2013 Search PubMed.
- E. Greenwald, J. M. Gordon and Y. Zarmi, Appl. Phys. Lett., 2012, 100, 143703 CrossRef.
- P. C. Gach, S. C. C. Shih, J. Sustarich, J. D. Keasling, N. J. Hillson, P. D. Adams and A. K. Singh, ACS Synth. Biol., 2016, 5, 426–433 CrossRef CAS PubMed.
- S. C. C. Shih, G. Goyal, P. W. Kim, N. Koutsoubelis, J. D. Keasling, P. D. Adams, N. J. Hillson and A. K. Singh, ACS Synth. Biol., 2015, 4, 1151–1164 CrossRef CAS PubMed.
- M. T. Guo, A. Rotem, J. a. Heyman and D. a. Weitz, Lab Chip, 2012, 12, 2146–2155 RSC.
- L. Liu, A. Pan, C. Spofford, N. Zhou and H. S. Alper, Metab. Eng., 2015, 29, 36–45 CrossRef CAS PubMed.
- J. Y. H. Kim, H. S. Kwak, Y. J. Sung, H. Il Choi, M. E. Hong, H. S. Lim, J.-H. Lee, S. Y. Lee and S. J. Sim, Sci. Rep., 2016, 6, 21155 CrossRef CAS PubMed.
- L. Hughes, Trends. Ecol. Evol., 2000, 15, 56–61 CrossRef PubMed.
- J. C. Orr, V. J. Fabry, O. Aumont, L. Bopp, S. C. Doney, R. A. Feely, A. Gnanadesikan, N. Gruber, A. Ishida, F. Joos, R. M. Key, K. Lindsay, E. Maier-Reimer, R. Matear, P. Monfray, A. Mouchet, R. G. Najjar, G.-K. Plattner, K. B. Rodgers, C. L. Sabine, J. L. Sarmiento, R. Schlitzer, R. D. Slater, I. J. Totterdell, M.-F. Weirig, Y. Yamanaka and A. Yool, Nature, 2005, 437, 681–686 CrossRef CAS PubMed.
- B. A. Bancroft, N. J. Baker and A. R. Blaustein, Ecol. Lett., 2007, 10, 332–345 CrossRef PubMed.
- E. S. Bernhardt, E. J. Rosi and M. O. Gessner, Front. Ecol. Environ., 2017, 15, 84–90 CrossRef.
- A. L. Andrady, Mar. Pollut. Bull., 2011, 62, 1596–1605 CrossRef CAS PubMed.
- A. R. Gunderson, E. J. Armstrong and J. H. Stillman, Ann. Rev. Mar. Sci., 2016, 8, 357–378 CrossRef PubMed.
- E. H. Heugens, A. J. Hendriks, T. Dekker, N. M. van Straalen and W. Admiraal, Crit. Rev. Toxicol., 2001, 31, 247–284 CrossRef CAS PubMed.
- C. M. Crain, K. Kroeker and B. S. Halpern, Ecol. Lett., 2008, 11, 1304–1315 CrossRef PubMed.
- F. J. R. C. Coelho, A. L. Santos, J. Coimbra, A. Almeida, Â. Cunha, D. F. R. Cleary, R. Calado and N. C. M. Gomes, Ecol. Evol., 2013, 3, 1808–1818 CrossRef PubMed.
- J.-M. Kim, K. Shin, K. Lee and B.-K. Park, Limnol. Oceanogr.: Methods, 2008, 6, 208–217 CrossRef CAS.
- H. S. Findlay, M. A. Kendall, J. I. Spicer, C. Turley and S. Widdicombe, Aquat. Biol., 2008, 3, 51–62 CrossRef.
- C. D. MacLeod, H. L. Doyle and K. I. Currie, Biogeosciences, 2015, 12, 713–721 CrossRef.
- L. J. Falkenberg, B. D. Russell and S. D. Connell, Limnol. Oceanogr.: Methods, 2016, 14, 278–291 CrossRef CAS.
- C. M. McGraw, C. E. Cornwall, M. R. Reid, K. I. Currie, C. D. Hepburn, P. Boyd, C. L. Hurd and K. a. Hunter, Limnol. Oceanogr.: Methods, 2010, 8, 686–694 CAS.
- U. Riebesell, J. Czerny, K. von Bröckel, T. Boxhammer, J. Büdenbender, M. Deckelnick, M. Fischer, D. Hoffmann, S. A. Krug, U. Lentz, A. Ludwig, R. Muche and K. G. Schulz, Biogeosciences, 2013, 10, 1835–1847 CrossRef.
- P. L. Jokiel, K. D. Bahr and K. S. Rodgers, Limnol. Oceanogr.: Methods, 2014, 12, 313–322 CrossRef.
- N. Ramanathan, O. Simakov, C. A. Merten, D. Arendt, V. Smith and J.-S. Hwang, PLoS One, 2015, 10, e0140553 Search PubMed.
- W. J. Palen, C. E. Williamson, A. A. Clauser and D. E. Schindler, Proc. R. Soc. B, 2005, 272(1569), 1227–1234 CrossRef PubMed.
- A. L. Santos, I. Henriques, N. C. M. Gomes, A. Almeida, A. Correia and A. Cunha, Aquat. Sci., 2011, 73, 63–77 CrossRef CAS.
- R. Przeslawski, A. R. Davis and K. Benkendorff, Glob. Chang. Biol., 2005, 11, 515–522 CrossRef.
- C. L. Searle, L. K. Belden, B. A. Bancroft, B. A. Han, L. M. Biga and A. R. Blaustein, Oecologia, 2010, 162, 237–245 CrossRef PubMed.
- D. McKee, D. Atkinson, S. Collings and J. Eaton, Freshw. Rev., 2000, 51–58 Search PubMed.
- J. J. C. Netten, E. H. van Nes, M. Scheffer and R. M. M. Roijackers, Limnol. Oceanogr.: Methods, 2008, 6, 223–229 CrossRef.
- E. E. Bockmon, C. A. Frieder, M. O. Navarro, L. A. White-Kershek and A. G. Dickson, Biogeosciences, 2013, 10, 5967–5975 CrossRef.
- J. Nouguier, B. Mostajir, E. Le Floc’h and F. Vidussi, Limnol. Oceanogr.: Methods, 2007, 5, 269–279 CrossRef.
- G. Brennan and S. Collins, Nat. Clim. Change, 2015, 5, 892–897 CrossRef.
- M. J. Behrenfeld, R. T. O'Malley, D. a. Siegel, C. R. McClain, J. L. Sarmiento, G. C. Feldman, A. J. Milligan, P. G. Falkowski, R. M. Letelier and E. S. Boss, Nature, 2006, 444, 752–755 CrossRef CAS PubMed.
- T. G. Benton, M. Solan, J. M. J. Travis and S. M. Sait, Trends. Ecol. Evol., 2007, 22, 516–521 CrossRef PubMed.
- R. D. Sagarin, J. Adams, C. A. Blanchette, R. C. Brusca, J. Chorover, J. E. Cole, F. Micheli, A. Munguia-Vega, C. M. Rochman, K. Bonine, J. van Haren and P. A. Troch, Front. Ecol. Environ., 2016, 14, 389–396 CrossRef.
- C. A. Oviatt, A. A. Keller, P. A. Sampou and L. L. Beatty, Mar. Ecol.: Prog. Ser., 1986, 28, 69–80 CrossRef.
- S. Nagarajan, S. K. Chou, S. Cao, C. Wu and Z. Zhou, Bioresour. Technol., 2013, 145, 150–156 CrossRef CAS PubMed.
- J. C. Quinn and R. Davis, Bioresour. Technol., 2015, 184, 444–452 CrossRef CAS PubMed.
- A. Sun, R. Davis, M. Starbuck, A. Ben-Amotz, R. Pate and P. T. Pienkos, Energy, 2011, 36, 5169–5179 CrossRef.
- L. Amer, B. Adhikari and J. Pellegrino, Bioresour. Technol., 2011, 102, 9350–9359 CrossRef CAS PubMed.
- J. Sheehan, T. Dunahay, J. Benemann and P. Roessler, A look back at the U.S. Department of Energy's aquatic species program: biodiesel from algae, 1998, vol. 328 Search PubMed.
- Transportation statistics annual report 2015, ed. W. Moore, 2015 Search PubMed.
- R. Slade and A. Bauen, Biomass Bioenergy, 2013, 53, 29–38 CrossRef.
- J. W. Richardson, M. D. Johnson and J. L. Outlaw, Algal Res., 2012, 1, 93–100 CrossRef.
- J. W. Richardson, M. D. Johnson, X. Zhang, P. Zemke, W. Chen and Q. Hu, Algal Res., 2014, 4, 96–104 CrossRef.
- R. Davis, A. Aden and P. T. Pienkos, Appl. Energy, 2011, 88, 3524–3531 CrossRef.
- L. B. Brentner, M. J. Eckelman and J. B. Zimmerman, Environ. Sci. Technol., 2011, 45, 7060–7067 CrossRef CAS PubMed.
- F. G. Acién Fernández, C. V. González-López, J. M. Fernández Sevilla and E. Molina Grima, Appl. Microbiol. Biotechnol., 2012, 96, 577–586 CrossRef PubMed.
- L. B. Brentner, J. Peccia and J. B. Zimmerman, Environ. Sci. Technol., 2010, 44, 2243–2254 CrossRef CAS PubMed.
- E. P. Resurreccion, L. M. Colosi, M. a. White and A. F. Clarens, Bioresour. Technol., 2012, 126, 298–306 CrossRef CAS PubMed.
- J. H. de Vree, R. Bosma, M. Janssen, M. J. Barbosa and R. H. Wijffels, Biotechnol. Biofuels, 2015, 8, 215 CrossRef PubMed.
- H. W. Kim, A. K. Marcus, J. H. Shin and B. E. Rittmann, Environ. Sci. Technol., 2011, 45, 5032–5038 CrossRef CAS PubMed.
- R. H. Wijffels and M. J. Barbosa, Science, 2010, 329, 796–799 CrossRef CAS PubMed.
- B. Wang, C. Q. Lan and M. Horsman, Biotechnol. Adv., 2012, 30, 904–912 CrossRef CAS PubMed.
- O. Pulz and K. Scheibenbogen, Adv. Biochem. Eng./Biotechnol., 1998, 59, 123–152 CAS.
- A. E. Eltayeb, O. Khalil, S. Al-Hallaj and F. Teymour, Comput. Chem. Eng., 2010, 34, 1323–1340 CrossRef CAS.
- M. Janssen, J. Tramper, L. R. Mur and R. H. Wijffels, Biotechnol. Bioeng., 2003, 81, 193–210 CrossRef CAS PubMed.
- E. Molina, J. Fernández, F. G. Acién and Y. Chisti, J. Biotechnol., 2001, 92, 113–131 CrossRef CAS.
- C. Posten, Eng. Life Sci., 2009, 9, 165–177 CrossRef CAS.
- J.-W. F. Zijffers, M. Janssen, J. Tramper and R. H. Wijffels, Mar. Biotechnol., 2008, 10, 404–415 CrossRef CAS PubMed.
- M. Iqbal, Introduction to Solar Radiation, Toronto, New York, 1983 Search PubMed.
- T. Kondo, T. Wakayama and J. Miyake, Int. J. Hydrogen Energy, 2006, 31, 1522–1526 CrossRef CAS.
- G. Chini Zittelli, R. Pastorelli and M. R. Tredici, J. Appl. Phycol., 2000, 12, 521–526 CrossRef.
- E. E. Jung, A. Jain, N. Voulis, D. F. R. Doud, L. T. Angenent and D. Erickson, Bioresour. Technol., 2014, 171, 495–499 CrossRef CAS PubMed.
- Y. Sun, Q. Liao, Y. Huang, A. Xia, Q. Fu, X. Zhu and Y. Zheng, Bioresour. Technol., 2016, 220, 215–224 CrossRef CAS PubMed.
- O. Pulz, N. Gerbsch and R. Buchholz, J. Appl. Phycol., 1995, 7, 145–149 CrossRef.
- C.-Y. Chen, C.-M. Lee and J.-S. Chang, Biochem. Eng. J., 2006, 32, 33–42 CrossRef CAS.
- C.-L. Guo, X. Zhu, Q. Liao, Y.-Z. Wang, R. Chen and D.-J. Lee, Bioresour. Technol., 2011, 102, 8507–8513 CrossRef CAS PubMed.
- J.-W. F. Zijffers, S. Salim, M. Janssen, J. Tramper and R. H. Wijffels, Chem. Eng. J., 2008, 145, 316–327 CrossRef CAS.
- S. S. Ahsan, B. Pereyra, E. E. Jung and D. Erickson, Opt. Express, 2014, 22, A1526 CrossRef PubMed.
- S. N. Genin, J. S. Aitchison and D. G. Allen, Algal Res., 2015, 12, 529–538 CrossRef.
- E. E. Jung, M. Kalontarov, D. F. R. Doud, M. D. Ooms, L. T. Angenent, D. Sinton and D. Erickson, Lab Chip, 2012, 12, 3740 RSC.
- M. D. Ooms, V. J. Sieben, S. C. Pierobon, E. E. Jung, M. Kalontarov, D. Erickson and D. Sinton, Phys. Chem. Chem. Phys., 2012, 14, 4817–4823 RSC.
- S. C. Pierobon, M. D. Ooms and D. Sinton, J. Micromech. Microeng., 2014, 24, 45017 CrossRef.
- Y. Sun, Y. Huang, Q. Liao, Q. Fu and X. Zhu, Bioresour. Technol., 2016, 207, 31–38 CrossRef CAS PubMed.
- M. D. Ooms, Y. Jeyaram and D. Sinton, Appl. Phys. Lett., 2015, 106, 63902 CrossRef.
- S. F. Mohsenpour, B. Richards and N. Willoughby, Bioresour. Technol., 2012, 125, 75–81 CrossRef CAS PubMed.
- L. Wondraczek, M. Batentschuk, M. a. Schmidt, R. Borchardt, S. Scheiner, B. Seemann, P. Schweizer and C. J. Brabec, Nat. Commun., 2013, 4, 2047 Search PubMed.
- H. Delavari Amrei, R. Ranjbar, S. Rastegar, B. Nasernejad and A. Nejadebrahim, J. Appl. Phycol., 2014, 67–74 Search PubMed.
- H. Delavari Amrei, B. Nasernejad, R. Ranjbar and S. Rastegar, J. Appl. Phycol., 2013, 26, 1493–1500 CrossRef.
- Q. Xia, M. Batentschuk, A. Osvet, P. Richter, D. P. Häder, J. Schneider, C. J. Brabec, L. Wondraczek and A. Winnacker, Opt. Express, 2013, 21(suppl. 6), A909–A916 CrossRef PubMed.
- S. F. Mohsenpour and N. Willoughby, Bioresour. Technol., 2013, 142, 147–153 CrossRef CAS PubMed.
- K. R. Menon, S. Jose and G. K. Suraishkumar, Biotechnol. J., 2014, 9, 1547–1553 CrossRef CAS PubMed.
- D. Parlevliet and N. R. Moheimani, Aquat. Biosyst., 2014, 10, 1–9 CrossRef PubMed.
- A. Mojiri, R. Taylor, E. Thomsen and G. Rosengarten, Renewable Sustainable Energy Rev., 2013, 28, 654–663 CrossRef.
- M. D. Redwood, R. Dhillon, R. L. Orozco, X. Zhang, D. J. Binks, M. Dickinson and L. E. Macaskie, Biotechnol. Lett., 2012, 34, 2229–2234 CrossRef CAS PubMed.
- A. Jacob, E. C. Bucharsky and K. GuenterSchell, J. Bioprocess. Biotech., 2012, 2, 1–8 Search PubMed.
- A. a. Tsygankov, Y. Hirata, M. Miyake, Y. Asada and J. Miyake, J. Ferment. Bioeng., 1994, 77, 575–578 CrossRef CAS.
- M. Heining, A. Sutor, S. C. Stute and C. P. Lindenberger, J. Appl. Phycol., 2015, 59–66 CrossRef CAS.
- M. Heining and R. Buchholz, Biotechnol. J., 2015, 10, 1131–1137 CrossRef CAS PubMed.
- E. Ono and J. L. Cuello, Energy, 2004, 29, 1651–1657 CrossRef CAS.
- J. Gordon, Int. J. Hydrogen Energy, 2002, 27, 1175–1184 CrossRef CAS.
- G. E. Arnaoutakis, J. Marques-Hueso, T. K. Mallick and B. S. Richards, Sol. Energy, 2013, 93, 235–243 CrossRef.
- D. Feuermann, J. M. Gordon and M. Huleihil, Sol. Energy, 2002, 72, 459–472 CrossRef.
- J. C. Ogbonna, T. Soejima and H. Tanaka, J. Biotechnol., 1999, 70, 289–297 CrossRef CAS PubMed.
- D. Dye, J. Muhs, B. Wood and R. Sims, J. Sol. Energy Eng., 2011, 133, 15001 CrossRef.
- G. C. Zittelli, N. Biondi and M. R. Tredici, Algae for Biofuels and Energy, Springer Netherlands, Dordrecht, 2013 Search PubMed.
- J. H. Karp, E. J. Tremblay and J. E. Ford, Opt. Express, 2010, 18, 1122–1133 CrossRef CAS PubMed.
- J. H. Karp, E. J. Tremblay, J. M. Hallas and J. E. Ford, Opt. Express, 2011, 19, A673–A685 CrossRef PubMed.
- P. Xie, H. Lin, Y. Liu and B. Li, Opt. Express, 2014, 22, A1389 CrossRef PubMed.
- S. Bouchard and S. Thibault, Appl. Opt., 2012, 51, 6848–6854 CrossRef PubMed.
- K. a. Baker, J. H. Karp, E. J. Tremblay, J. M. Hallas and J. E. Ford, Appl. Opt., 2012, 51, 1086–1094 CrossRef CAS PubMed.
- N. S. Lewis, Science, 2007, 315, 798 CrossRef CAS PubMed.
- T. M. Sobczuk, F. G. Camacho, F. C. Rubio, F. G. A. Fernández and E. M. Grima, Biotechnol. Bioeng., 2000, 67, 465–475 CrossRef CAS.
- G. T. B. Norddahl, Bioprocess Biosyst. Eng., 2016, 39, 613–626 CrossRef PubMed.
- A. P. Carvalho and F. X. Malcata, Biotechnol. Prog., 2001, 17, 265–272 CrossRef CAS PubMed.
- H. Schlichting and K. Gersten, Boundary layer theory, Springer-Verlag, Berlin, 8th edn, 2000 Search PubMed.
- K. Zhang, N. Kurano and S. Miyachi, Bioprocess Biosyst. Eng., 2002, 25, 97–101 CrossRef CAS PubMed.
- H. J. Ryu, K. K. Oh and Y. S. Kim, J. Ind. Eng. Chem., 2009, 15, 471–475 CrossRef CAS.
- I. De Godos, J. L. Mendoza, F. G. Acién, E. Molina, C. J. Banks, S. Heaven and F. Rogalla, Bioresour. Technol., 2014, 153, 307–314 CrossRef CAS PubMed.
- F. Camacho Rubio, F. G. Acién Fernández, J. a. Sánchez Pérez, F. García Camacho and E. Molina Grima, Biotechnol. Bioeng., 1999, 62, 71–86 CrossRef.
- F. G. Acién, J. M. Fernández, J. J. Magán and E. Molina, Biotechnol. Adv., 2012, 30, 1344–1353 CrossRef PubMed.
- Q. Hu, N. Kurano, M. Kawachi, I. Iwasaki and S. Miyachi, Appl. Microbiol. Biotechnol., 1998, 49, 655–662 CrossRef CAS.
- A. Kumar, X. Yuan, A. K. Sahu, J. Dewulf, S. J. Ergas and H. Van Langenhove, J. Chem. Technol. Biotechnol., 2010, 85, 387–394 CrossRef CAS.
- R. Sayre, Bioscience, 2010, 60, 722–727 CrossRef.
- M. K. Lam, K. T. Lee and A. R. Mohamed, Int. J. Greenhouse Gas Control, 2012, 10, 456–469 CrossRef CAS.
- K. L. Kadam, Energy Convers. Manage., 1997, 38, 505–510 CrossRef.
- S. P. Cuellar-Bermudez, J. S. Garcia-Perez, B. E. Rittmann and R. Parra-Saldivar, J. Cleaner Prod., 2015, 98, 53–65 CrossRef CAS.
- G. Olivieri, P. Salatino and A. Marzocchella, J. Chem. Technol. Biotechnol., 2014, 89, 178–195 CrossRef CAS.
- L. Zhang, L. Chen, J. Wang, Y. Chen, X. Gao, Z. Zhang and T. Liu, Bioresour. Technol., 2015, 181, 136–142 CrossRef CAS PubMed.
- W. B. Zimmerman, M. Zandi, H. C. H. Bandulasena, V. Tesar, D. J. Gilmour and K. Ying, Appl. Energy, 2011, 88, 3357–3369 CrossRef CAS.
- M. Grammatika and W. B. Zimmerman, Dynamics of Atmospheres and Oceans, 2001, 34, 327–348 CrossRef.
- R. Parmar and S. K. Majumder, Asia-Pac. J. Chem. Eng., 2015, 450–465 CrossRef CAS.
- K. Ying, M. K. H. Al-mashhadani, J. O. Hanotu, D. J. Gilmour and W. B. Zimmerman, Engineering, 2013, 2013, 735–743 CrossRef.
- W. B. Zimmerman, B. N. Hewakandamby and V. Tesă, Food Bioprod. Process., 2009, 7, 215–227 CrossRef.
- L. Merriman, A. Moix, R. Beitle and J. Hestekin, Chem. Eng. J., 2014, 253, 165–173 CrossRef CAS.
- T. J. Rector, J. L. Garland and S. O. Starr, J. Membr. Sci., 2006, 278, 144–150 CrossRef CAS.
- G. Peterat, H. Schmolke, T. Lorenz, A. Llobera, D. Rasch, C. Klages and R. Krull, Biotechnol. Bioeng., 2014, 111, 1809–1819 CrossRef CAS PubMed.
- L.-H. Fan, Y.-T. Zhang, L. Zhang and H.-L. Chen, J. Membr. Sci., 2008, 325, 336–345 CrossRef CAS.
- X. Li and L. Zu, Chem. Eng. Technol., 2016, 945–952 CrossRef CAS.
- Y. Chisti, Biotechnol. Adv., 2007, 25, 294–306 CrossRef CAS PubMed.
- S. Ojanen, E. Tyystjärvi, H. Holmberg and P. Ahtila, J. Appl. Phycol., 2015, 27, 1169–1175 CrossRef CAS.
- E. Molina Grima, F. G. Acién Fernández, F. García Camacho, Y. Chisti, F. G. Acien Farnandez, F. Garcia Camacho, Y. Chisti, F. G. A. Fernández, F. García Camacho, E. M. Grima, F. G. Acie and Y. Chisti, J. Biotechnol., 1999, 70, 231–247 CrossRef CAS.
- L. Xu, P. J. Weathers, X.-R. Xiong and C.-Z. Liu, Eng. Life Sci., 2009, 9, 178–189 CrossRef CAS.
- S. Judd, The MBR Book: Principles and Applications of Membrane Bioreactors for Water and Wastewater Treatment, 2nd edn, 2011 Search PubMed.
- M. S. A. Rahaman, L.-H. Cheng, X.-H. Xu, L. Zhang and H.-L. Chen, Renewable Sustainable Energy Rev., 2011, 15, 4002–4012 CrossRef.
- A. Gabelman and S.-T. Hwang, J. Membr. Sci., 1999, 159, 61–106 CrossRef CAS.
- H. A. Rangwala, J. Membr. Sci., 1996, 229–240 CrossRef CAS.
- B. S. Ferreira, H. L. Fernandes, A. Reis and M. Mateus, J. Chem. Technol. Biotechnol., 1998, 71, 61–70 CrossRef CAS.
- L.-H. Fan, Y.-T. Zhang, L.-H. Cheng, L. Zhang, D.-S. Tang and H.-L. Chen, Chem. Eng. Technol., 2007, 30, 1094–1099 CrossRef CAS.
- L. Cheng, L. Zhang, H. Chen and C. Gao, Sep. Purif. Technol., 2006, 50, 324–329 CrossRef CAS.
- A. Jain, N. Voulis, E. E. Jung, D. F. R. Doud, W. B. Miller, L. T. Angenent and D. Erickson, Environ. Sci. Technol., 2015, 49, 6327–6334 CrossRef CAS PubMed.
- S. S. Ahsan, A. Gumus, A. Jain, L. T. Angenent and D. Erickson, Bioresour. Technol., 2015, 192, 845–849 CrossRef CAS PubMed.
- M. Kalontarov, D. F. R. Doud, E. E. Jung, L. T. Angenent, D. Erickson, E. E. Jung, L. T. Angenent and D. Erickson, RSC Adv., 2014, 4, 1460–1468 RSC.
- M. R. Bilad, H. A. Arafat and I. F. J. Vankelecom, Biotechnol. Adv., 2014, 32, 1283–1300 CrossRef CAS PubMed.
- S. a. Markov, M. J. Bazin and D. O. Hall, Enzyme Microb. Technol., 1995, 17, 306–310 CrossRef CAS.
- S. C. Pierobon, J. Riordon, B. Nguyen and D. Sinton, Bioresour. Technol., 2016, 209, 391–396 CrossRef CAS PubMed.
- V. Vasudevan, R. W. Stratton, M. N. Pearlson, G. R. Jersey, A. G. Beyene, J. C. Weissman, M. Rubino and J. I. Hileman, Environ. Sci. Technol., 2012, 46, 2451–2459 CrossRef CAS PubMed.
- R. Davis, J. Markham, C. Kinchin, N. Grundl, E. C. D. Tan and D. Humbird, Process Design and Economics for the Production of Algal Biomass: Algal Biomass Production in Open Pond Systems and Processing Through Dewatering for Downstream Conversion, NREL/TP-5100-64772, 2016, http://www.nrel.gov/docs/fy16osti/64772.pdf Search PubMed.
- O. Jorquera, A. Kiperstok, E. a. Sales, M. Embiruçu and M. L. Ghirardi, Bioresour. Technol., 2010, 101, 1406–1413 CrossRef CAS PubMed.
- K. Heimann, Curr. Opin. Biotechnol., 2016, 38, 183–189 CrossRef CAS PubMed.
- B. Podola, T. Li and M. Melkonian, Trends Biotechnol., 2017, 35 DOI:10.1016/j.tibtech.2016.06.004.
- T. Liu, J. Wang, Q. Hu, P. Cheng, B. Ji, J. Liu, Y. Chen, W. Zhang, X. Chen, L. Chen, L. Gao, C. Ji and H. Wang, Bioresour. Technol., 2013, 127, 216–222 CrossRef CAS PubMed.
- B. Podola, T. Li and M. Melkonian, Trends Biotechnol., 2017, 35, 121–132 CrossRef CAS PubMed.
- T. Li, B. Piltz, B. Podola, A. Dron, D. de Beer and M. Melkonian, Biotechnol. Bioeng., 2016, 113, 1046–1055 CrossRef CAS PubMed.
- T. Naumann, Z. Çebi, B. Podola and M. Melkonian, J. Appl. Phycol., 2013, 25, 1413–1420 CrossRef CAS.
- E. Nowack, B. Podola and M. Melkonian, Protist, 2005, 156, 239–251 CrossRef PubMed.
- P. J. Schnurr and D. G. Allen, Renewable Sustainable Energy Rev., 2015, 52, 418–429 CrossRef CAS.
- D. Hoh, S. Watson and E. Kan, Chem. Eng. J., 2016, 287, 466–473 CrossRef CAS.
- R. Muñoz, C. Köllner and B. Guieysse, J. Hazard. Mater., 2009, 161, 29–34 CrossRef PubMed.
- M. Kesaano, R. D. Gardner, K. Moll, E. Lauchnor, R. Gerlach, B. M. Peyton and R. C. Sims, Bioresour. Technol., 2015, 180, 7–15 CrossRef CAS PubMed.
- L. Katarzyna, G. Sai and O. Avijeet Singh, Renewable Sustainable Energy Rev., 2015, 42, 1418–1427 CrossRef CAS.
- S. N. Genin, J. Stewart Aitchison and D. Grant Allen, Bioresour. Technol., 2014, 155, 136–143 CrossRef CAS PubMed.
- M. Gross, D. Jarboe and Z. Wen, Appl. Microbiol. Biotechnol., 2015, 99, 5781–5789 CrossRef CAS PubMed.
- P. J. Schnurr, G. S. Espie and D. G. Allen, Bioresour. Technol., 2013, 136, 337–344 CrossRef CAS PubMed.
- S. H. Lee, H. M. Oh, B. H. Jo, S. A. Lee, S. Y. Shin, H. S. Kim, S. H. Lee and C. Y. Ahn, J. Microbiol. Biotechnol., 2014, 24, 1566–1573 CrossRef CAS PubMed.
- L. Christenson and R. Sims, Biotechnol. Adv., 2011, 29, 686–702 CrossRef CAS PubMed.
- L. B. Christenson and R. C. Sims, Biotechnol. Bioeng., 2012, 109, 1674–1684 CrossRef CAS PubMed.
- M. B. Johnson and Z. Wen, Appl. Microbiol. Biotechnol., 2010, 85, 525–534 CrossRef CAS PubMed.
- H. C. Bernstein, M. Kesaano, K. Moll, T. Smith, R. Gerlach, R. P. Carlson, C. D. Miller, B. M. Peyton, K. E. Cooksey, R. D. Gardner and R. C. Sims, Bioresour. Technol., 2014, 156, 206–215 CrossRef CAS PubMed.
- M. Gross, W. Henry, C. Michael and Z. Wen, Bioresour. Technol., 2013, 150, 195–201 CrossRef CAS PubMed.
- W. Blanken, M. Janssen, M. Cuaresma, Z. Libor, T. Bhaiji and R. H. Wijffels, Biotechnol. Bioeng., 2014, 111, 2436–2445 CrossRef CAS PubMed.
- M. Gross and Z. Wen, Bioresour. Technol., 2014, 171, 50–58 CrossRef CAS PubMed.
- J. Shi, B. Podola and M. Melkonian, J. Appl. Phycol., 2007, 19, 417–423 CrossRef CAS.
- B. Podola and M. Melkonian, J. Appl. Phycol., 2003, 15, 415–424 CrossRef CAS.
- R. M. Benstein, Z. Çebi, B. Podola and M. Melkonian, Mar. Biotechnol., 2014, 16, 621–628 CrossRef CAS PubMed.
- L. K. P. Schultze, M. V. Simon, T. Li, D. Langenbach, B. Podola and M. Melkonian, Algal Res., 2015, 8, 37–44 CrossRef.
- P. Cheng, J. Wang and T. Liu, Bioresour. Technol., 2014, 166, 527–533 CrossRef CAS PubMed.
- T. E. Murphy and H. Berberoglu, Biotechnol. Prog., 2014, 30, 348–359 CrossRef CAS PubMed.
- M. Gross, V. Mascarenhas and Z. Wen, Biotechnol. Bioeng., 2015, 112, 2040–2050 CrossRef CAS PubMed.
- A. Ozkan, K. Kinney, L. Katz and H. Berberoglu, Bioresour. Technol., 2012, 114, 542–548 CrossRef CAS PubMed.
- S. Yin, J. Wang, L. Chen and T. Liu, Biotechnol. Lett., 2015, 37, 1819–1827 CrossRef CAS PubMed.
- J. Shi, B. Podola and M. Melkonian, Bioresour. Technol., 2014, 154, 260–266 CrossRef CAS PubMed.
- P. Cheng, B. Ji, L. Gao, W. Zhang, J. Wang and T. Liu, Bioresour. Technol., 2013, 138, 95–100 CrossRef CAS PubMed.
- B. Ji, W. Zhang, N. Zhang, J. Wang, G. A. Lutzu and T. Liu, Bioprocess Biosyst. Eng., 2014, 37, 1369–1375 CrossRef CAS PubMed.
- T. M. Mata, A. A. A. Martins and N. S. Caetano, Renewable Sustainable Energy Rev., 2010, 14, 217–232 CrossRef CAS.
- F. Berner, K. Heimann and M. Sheehan, J. Appl. Phycol., 2015, 27, 1793–1804 CrossRef CAS.
- J. Wang, J. Liu and T. Liu, Biotechnol. Biofuels, 2015, 8, 49 CrossRef PubMed.
- C. Ji, J. Wang, W. Zhang, J. Liu, H. Wang, L. Gao and T. Liu, J. Appl. Phycol., 2014, 26, 173–180 CrossRef CAS.
- T. Li, B. Podola and M. Melkonian, Algal Res., 2016, 13, 30–40 CrossRef.
- T. Li, B. Podola, D. de Beer and M. Melkonian, J. Microbiol. Methods, 2015, 117, 100–107 CrossRef CAS PubMed.
- L. E. de-Bashan and Y. Bashan, Bioresour. Technol., 2010, 101, 1611–1627 CrossRef CAS PubMed.
- N. Mallick, BioMetals, 2002, 15, 377–390 CrossRef CAS PubMed.
- C. A. Santos and A. Reis, Appl. Microbiol. Biotechnol., 2014, 98, 5839–5846 CrossRef CAS PubMed.
- I. Moreno-Garrido, Bioresour. Technol., 2008, 99, 3949–3964 CrossRef CAS PubMed.
- L. E. de-Bashan, Y. Bashan, M. Moreno, V. K. Lebsky and J. J. Bustillos, Can. J. Microbiol., 2002, 48, 514–521 CrossRef CAS PubMed.
- S. a. Covarrubias, L. E. De-Bashan, M. Moreno and Y. Bashan, Appl. Microbiol. Biotechnol., 2012, 93, 2669–2680 CrossRef CAS PubMed.
- S. C. Pierobon, J. Riordon, B. Nguyen, M. D. Ooms and D. Sinton, Biotechnol. Bioeng., 2017, 114, 2023–2031 CrossRef CAS PubMed.
- P. S. Lau, N. F. Y. Tam and Y. S. Wong, Environ. Technol., 1997, 18, 945–951 CrossRef CAS.
- M. V. Jiménez-Pérez, P. Sánchez-Castillo, O. Romera, D. Fernández-Moreno and C. Pérez-Martínez, Enzyme Microb. Technol., 2004, 34, 392–398 CrossRef.
- Y. Singh, J. Microbiol. Biotechnol., 2003, 13, 687–691 CAS.
- S. N. Kosourov and M. Seibert, Biotechnol. Bioeng., 2009, 102, 50–58 CrossRef CAS PubMed.
- L. Pane, M. Feletti, C. Bertino and A. Carli, Aquacult. Int., 1998, 6, 411–420 CrossRef.
- L. E. De-Bashan, X. Mayali, B. M. Bebout, P. K. Weber, A. M. Detweiler, J.-P. Hernandez, L. Prufert-bebout and Y. Bashan, Algal Res., 2016, 15, 179–186 CrossRef.
- F. Krujatz, A. Lode, S. Brüggemeier, K. Schütz, J. Kramer, T. Bley, M. Gelinsky and J. Weber, Eng. Life Sci., 2015, 15, 678–688 CrossRef CAS.
- A. Lode, F. Krujatz, S. Brüggemeier, M. Quade, K. Schütz, S. Knaack, J. Weber, T. Bley and M. Gelinsky, Eng. Life Sci., 2015, 15, 177–183 CrossRef CAS.
- M. K. Lam and K. T. Lee, Biotechnol. Adv., 2012, 30, 673–690 CrossRef CAS PubMed.
- X. Chen, L. Hu, R. Xing, S. Liu, H. Yu, Y. Qin, K. Li, R. Li and P. Li, Eur. J. Lipid Sci. Technol., 2015, 117, 1192–1198 CrossRef CAS.
- A. I. Barros, A. L. Gonçalves, M. Simões and J. C. M. Pires, Renewable Sustainable Energy Rev., 2015, 41, 1489–1500 CrossRef.
- DOE (U.S. Department of Energy), Natl. Algal Biofuels Technol. Rev., 2016 Search PubMed.
- J. E. Coons, D. M. Kalb, T. Dale and B. L. Marrone, Algal Res., 2014, 6, 250–270 CrossRef.
- J. J. Milledge and S. Heaven, Rev. Environ. Sci. Bio/Technol., 2013, 12, 165–178 CrossRef.
- N. Pragya, K. K. Pandey and P. K. Sahoo, Renewable Sustainable Energy Rev., 2013, 24, 159–171 CrossRef CAS.
- J. Kim, G. Yoo, H. Lee, J. Lim, K. Kim, C. W. Kim, M. S. Park and J. Yang, Biotechnol. Adv., 2013, 31, 862–876 CrossRef CAS PubMed.
- T. Ndikubwimana, J. Chang, Z. Xiao, W. Shao, X. Zeng, I.-S. Ng and Y. Lu, Biotechnol. J., 2016, 11, 315–326 CrossRef CAS PubMed.
- X. Zhang, L. Wang, M. Sommerfeld and Q. Hu, Biomass Bioenergy, 2016, 93, 43–49 CrossRef CAS.
- T. E. Irving and D. G. Allen, Appl. Microbiol. Biotechnol., 2011, 92, 283–294 CrossRef CAS PubMed.
- K. Sander and G. S. Murthy, Int. J. Life Cycle Assess., 2010, 15, 704–714 CrossRef CAS.
- M. K. Weschler, W. J. Barr, W. F. Harper and A. E. Landis, Bioresour. Technol., 2014, 153, 108–115 CrossRef CAS PubMed.
- T.-S. Sim, A. Goh and E. W. Becker, Biomass, 1988, 16, 51–62 CrossRef.
- E. Molina Grima, E.-H. Belarbi, F. Acién Fernández, A. Robles Medina and Y. Chisti, Biotechnol. Adv., 2003, 20, 491–515 CrossRef CAS PubMed.
- G. Panis and J. R. Carreon, Algal Res., 2016, 18, 175–190 CrossRef.
- J. Prakash, B. Pushparaj, P. Carlozzi, G. Torzillo, E. Montaini and R. Materassi, Int. J. Sol. Energy, 1997, 18, 303–311 CrossRef.
- A. Fudholi, K. Sopian, M. Y. Othman and M. H. Ruslan, Energ. Build., 2014, 68, 121–129 CrossRef.
- J. Brink and S. Marx, Fuel, 2013, 106, 67–71 CrossRef CAS.
- K.-Y. Show, D.-J. Lee, J.-H. Tay, T.-M. Lee and J.-S. Chang, Bioresour. Technol., 2015, 184, 258–266 CrossRef CAS PubMed.
- J. Y. Lee, C. Yoo, S. Y. Jun, C. Y. Ahn and H. M. Oh, Bioresour. Technol., 2010, 101, S75–S77 CrossRef CAS PubMed.
- V. Pasquet, J.-R. Chérouvrier, F. Farhat, V. Thiéry, J.-M. Piot, J.-B. Bérard, R. Kaas, B. Serive, T. Patrice, J.-P. Cadoret and L. Picot, Process Biochem., 2011, 46, 59–67 CrossRef CAS.
- J. Iqbal and C. Theegala, Algal Res., 2013, 2, 34–42 CrossRef.
- J. Pan, T. Muppaneni, Y. Sun, H. K. Reddy, J. Fu, X. Lu and S. Deng, Fuel, 2016, 178, 49–55 CrossRef CAS.
- H. Yen, S. Yang, C. Chen and J. Chang, Bioresour. Technol., 2015, 184, 291–296 CrossRef CAS PubMed.
- R. L. Mendes, B. P. Nobre, M. T. Cardoso, A. P. Pereira and A. F. Palavra, Inorg. Chim. Acta, 2003, 356, 328–334 CrossRef CAS.
- S. Machmudah, A. Shotipruk, M. Goto, M. Sasaki and T. Hirose, Ind. Eng. Chem. Res., 2006, 45, 3652–3657 CrossRef CAS.
- G. Brunner, J. Food Eng., 2005, 67, 21–33 CrossRef.
- P. P. G. Jessop, D. J. D. Heldebrant, X. Li, C. A. C. Eckert and C. C. L. Liotta, Nature, 2005, 436, 1102 CrossRef CAS PubMed.
- B. E. Richter, B. a. Jones, J. L. Ezzell, N. L. Porter, N. Avdalovic and C. Pohl, Anal. Chem., 1996, 68, 1033–1039 CrossRef CAS.
- M. A. Hejazi, C. de Lamarliere, J. M. S. Rocha, M. Vermuë, J. Tramper and R. H. Wijffels, Biotechnol. Bioeng., 2002, 79, 29–36 CrossRef CAS PubMed.
- R. B. Levine, T. Pinnarat and P. E. Savage, Energy Fuels, 2010, 24, 5235–5243 CrossRef CAS.
- E. S. Umdu, M. Tuncer and E. Seker, Bioresour. Technol., 2009, 100, 2828–2831 CrossRef CAS PubMed.
- N. Nagle and P. Lemke, Appl. Biochem. Biotechnol., 1990, 24–25, 355–361 CrossRef CAS.
- R. Huerlimann, R. de Nys and K. Heimann, Biotechnol. Bioeng., 2010, 107, 245–257 CrossRef CAS PubMed.
- D.-T. Tran, K.-L. Yeh, C.-L. Chen and J.-S. Chang, Bioresour. Technol., 2012, 108, 119–127 CrossRef CAS PubMed.
- J.-Q. Lai, Z.-L. Hu, P.-W. Wang and Z. Yang, Fuel, 2012, 95, 329–333 CrossRef CAS.
- L. Fjerbaek, K. V. Christensen and B. Norddahl, Biotechnol. Bioeng., 2009, 102, 1298–1315 CrossRef CAS PubMed.
- B. Smith, H. C. Greenwell and A. Whiting, Energy Environ. Sci., 2009, 2, 262–271 CAS.
- N. H. Tran, J. R. Bartlett, G. S. K. Kannangara, A. S. Milev, H. Volk and M. A. Wilson, Fuel, 2010, 89, 265–274 CrossRef CAS.
- T. A. Milne, R. J. Evans and N. Nagle, Biomass, 1990, 21, 219–232 CrossRef CAS.
- H.-W. Yen, I.-C. Hu, C.-Y. Chen, S.-H. Ho, D.-J. Lee and J.-S. Chang, Bioresour. Technol., 2013, 135, 166–174 CrossRef CAS PubMed.
- A. Banerjee, R. Sharma, Y. Chisti and U. C. Banerjee, Crit. Rev. Biotechnol., 2002, 22, 245–279 CrossRef CAS PubMed.
- L. Soh and J. Zimmerman, Green Chem., 2011, 13, 1422 RSC.
- S. Wang, X. M. Jiang, X. X. Han and J. G. Liu, Energy Fuels, 2009, 23, 5173–5178 CrossRef CAS.
- X. Miao, Q. Wu and C. Yang, J. Anal. Appl. Pyrolysis, 2004, 71, 855–863 CrossRef CAS.
- X. Cheng, M. D. Ooms and D. Sinton, Lab Chip, 2016, 16, 256–260 RSC.
- D. C. Elliott, T. R. Hart, A. J. Schmidt, G. G. Neuenschwander, L. J. Rotness, M. V. Olarte, A. H. Zacher, K. O. Albrecht, R. T. Hallen and J. E. Holladay, Algal Res., 2013, 2, 445–454 CrossRef.
- J. L. Faeth, P. J. Valdez and P. E. Savage, Energy Fuels, 2013, 27, 1391–1398 CrossRef CAS.
- Y. Zhou, L. Schideman, G. Yu and Y. Zhang, Energy Environ. Sci., 2013, 6, 3765 CAS.
- Y. Lu and P. E. Savage, J. Supercrit. Fluids, 2015, 99, 88–94 CrossRef CAS.
- G. Caputo, M. Dispenza, P. Rubio, F. Scargiali, G. Marotta and A. Brucato, J. Supercrit. Fluids, 2016, 118, 163–170 CrossRef CAS.
- L. Tiong, M. Komiyama, Y. Uemura and T. T. Nguyen, J. Supercrit. Fluids, 2016, 107, 408–413 CrossRef CAS.
- L. M. L. Laurens, N. Nagle, R. Davis, N. Sweeney, S. Van Wychen, A. Lowell and P. T. Pienkos, Green Chem., 2015, 17, 1145–1158 RSC.
- T. Dong, E. P. Knoshaug, R. Davis, L. M. L. Laurens, S. Van Wychen, P. T. Pienkos and N. Nagle, Algal Res., 2016, 19, 316–323 CrossRef.
- J. T. Ellis, N. N. Hengge, R. C. Sims and C. D. Miller, Bioresour. Technol., 2012, 111, 491–495 CrossRef CAS PubMed.
- C. Y. Chen, X. Q. Zhao, H. W. Yen, S. H. Ho, C. L. Cheng, D. J. Lee, F. W. Bai and J. S. Chang, Biochem. Eng. J., 2013, 78, 1–10 CrossRef CAS.
- S. H. Ho, S. W. Huang, C. Y. Chen, T. Hasunuma, A. Kondo and J. S. Chang, Bioresour. Technol., 2013, 135, 191–198 CrossRef CAS PubMed.
- J. R. Miranda, P. C. Passarinho and L. Gouveia, Bioresour. Technol., 2012, 104, 342–348 CrossRef CAS PubMed.
- R. Harun, M. K. Danquah and G. M. Forde, J. Chem. Technol. Biotechnol., 2010, 85, 199–203 CAS.
- H. Yen and D. Brune, Bioresour. Technol., 2007, 98, 130–134 CrossRef CAS PubMed.
- P. Bohutskyi, D. C. Kligerman, N. Byers, L. K. Nasr, C. Cua, S. Chow, C. Su, Y. Tang, M. J. Betenbaugh and E. J. Bouwer, Algal Res., 2016, 19, 278–290 CrossRef.
- A. Mahdy, I. A. Fotidis, E. Mancini, M. Ballesteros, C. González-Fernández and I. Angelidaki, Bioresour. Technol., 2017, 225, 272–278 CrossRef CAS PubMed.
- C. A. Henard, H. Smith, N. Dowe, M. G. Kalyuzhnaya, P. T. Pienkos and M. T. Guarnieri, Sci. Rep., 2016, 6, 21585 CrossRef CAS PubMed.
- J. Myung, W. M. Galega, J. D. Van Nostrand, T. Yuan, J. Zhou and C. S. Criddle, Bioresour. Technol., 2015, 198, 811–818 CrossRef CAS PubMed.
- J. Jin, C. Dupré, K. Yoneda, M. M. Watanabe, J. Legrand and D. Grizeau, Process Biochem., 2016, 51, 1866–1875 CrossRef CAS.
- Y. Michinaka, J. Biosci. Bioeng., 2003, 95, 435–440 CrossRef CAS PubMed.
- N. R. Moheimani, H. Matsuura, M. M. Watanabe and M. a. Borowitzka, J. Appl. Phycol., 2014, 26, 1453–1463 CrossRef CAS.
- P. Bombelli, T. Müller, T. W. Herling, C. J. Howe and T. P. J. Knowles, Adv. Energy Mater., 2015, 5, 1401299 CrossRef PubMed.
- N. Rashid, Y.-F. Cui, M. Saif Ur Rehman and J.-I. Han, Sci. Total Environ., 2013, 456–457, 91–94 CrossRef CAS PubMed.
- E. E. Powell, M. L. Mapiour, R. W. Evitts and G. A. Hill, Bioresour. Technol., 2009, 100, 269–274 CrossRef CAS PubMed.
- A. Anderson, A. Laohavisit, I. K. Blaby, P. Bombelli, C. J. Howe, S. S. Merchant, J. M. Davies and A. G. Smith, Plant Biotechnol. J., 2016, 14, 22–28 CrossRef CAS PubMed.
- S. Kondaveeti, K. S. Choi, R. Kakarla and B. Min, Front. Environ. Sci. Eng., 2014, 8, 784–791 CrossRef CAS.
- Y. Cui, N. Rashid, N. Hu, M. S. U. Rehman and J.-I. Han, Energy Convers. Manage., 2014, 79, 674–680 CrossRef CAS.
- P. Bombelli, R. W. Bradley, A. M. Scott, A. J. Philips, A. J. McCormick, S. M. Cruz, A. Anderson, K. Yunus, D. S. Bendall, P. J. Cameron, J. M. Davies, A. G. Smith, C. J. Howe and A. C. Fisher, Energy Environ. Sci., 2011, 4, 4690 CAS.
- N. Samsonoff, M. D. Ooms and D. Sinton, Appl. Phys. Lett., 2014, 104, 43704 CrossRef.
- R. W. Bradley, P. Bombelli, S. J. L. Rowden and C. J. Howe, Biochem. Soc. Trans., 2012, 40, 1302–1307 CrossRef CAS PubMed.
- L. Xu, D. W. F. Wim Brilman, J. a M. Withag, G. Brem and S. Kersten, Bioresour. Technol., 2011, 102, 5113–5122 CrossRef CAS PubMed.
- D. L. Sills, V. Paramita, M. J. Franke, M. C. Johnson, T. M. Akabas, C. H. Greene and J. W. Tester, Environ. Sci. Technol., 2013, 47, 687–694 CrossRef CAS PubMed.
- R. Saidur, E. A. Abdelaziz, A. Demirbas, M. S. Hossain and S. Mekhilef, Renewable Sustainable Energy Rev., 2011, 15, 2262–2289 CrossRef CAS.
- A. Demirbaş, Energy Convers. Manage., 2001, 42, 1357–1378 CrossRef.
- J. J. Milledge and S. Heaven, Rev. Environ. Sci. Bio/Technol., 2014, 13, 301–320 CrossRef CAS.
- G. Kumar, S. Shobana, W.-H. Chen, Q.-V. Bach, S.-H. Kim, A. E. Atabani and J.-S. Chang, Green Chem., 2017, 19, 44–67 RSC.
- W.-H. Chen, B.-J. Lin, M.-Y. Huang and J.-S. Chang, Bioresour. Technol., 2015, 184, 314–327 CrossRef CAS PubMed.
- D. Carpenter, T. L. Westover, S. Czernik and W. Jablonski, Green Chem., 2014, 16, 384–406 RSC.
- C. Tian, B. Li, Z. Liu, Y. Zhang and H. Lu, Renewable Sustainable Energy Rev., 2014, 38, 933–950 CrossRef CAS.
- H. Huang and X. Yuan, Prog. Energy Combust. Sci., 2015, 49, 59–80 CrossRef.
- C. Xu and N. Lad, Energy Fuels, 2008, 22, 635–642 CrossRef CAS.
- P. Biller and A. B. Ross, Bioresour. Technol., 2011, 102, 215–225 CrossRef CAS PubMed.
- D. C. Hietala and P. E. Savage, Chem. Eng. J., 2015, 265, 129–137 CrossRef CAS.
- P. Biller, B. K. Sharma, B. Kunwar and A. B. Ross, Fuel, 2015, 159, 197–205 CrossRef CAS.
- Q. V. Bach, M. V. Sillero, K. Q. Tran and J. Skjermo, Algal Res., 2014, 6, 271–276 CrossRef.
- C. Miao, M. Chakraborty and S. Chen, Bioresour. Technol., 2012, 110, 617–627 CrossRef CAS PubMed.
- S. M. Heilmann, H. T. Davis, L. R. Jader, P. A. Lefebvre, M. J. Sadowsky, F. J. Schendel, M. G. von Keitz and K. J. Valentas, Biomass Bioenergy, 2010, 34, 875–882 CrossRef CAS.
- J. Mumme, L. Eckervogt, J. Pielert, M. Diakité, F. Rupp and J. Kern, Bioresour. Technol., 2011, 102, 9255–9260 CrossRef CAS PubMed.
- B. Patel, M. Guo, A. Izadpanah, N. Shah and K. Hellgardt, Bioresour. Technol., 2016, 199, 288–299 CrossRef CAS PubMed.
- H. S. Kambo and A. Dutta, Energy Convers. Manage., 2015, 105, 746–755 CrossRef CAS.
- V. S. Sikarwar, M. Zhao, P. Clough, J. Yao, X. Zhong, M. Z. Memon, N. Shah, E. J. Anthony and P. S. Fennell, Energy Environ. Sci., 2016, 9, 2939–2977 CAS.
- K. Okabe, K. Murata, M. Nakanishi, T. Ogi, M. Nurunnabi and Y. Liu, Catal. Lett., 2009, 128, 171–176 CrossRef CAS.
- Y. Yang, H. Xiang, R. Zhang, B. Zhong and Y. Li, Catal. Today, 2005, 106, 170–175 CrossRef CAS.
- C. Cao, J. Hu, S. Li, W. Wilcox and Y. Wang, Catal. Today, 2009, 140, 149–156 CrossRef CAS.
- A. Hirano, K. Hon-Nami, S. Kunito, M. Hada and Y. Ogushi, Catal. Today, 1998, 45, 399–404 CrossRef CAS.
- Y. Lu, R. B. Levine and P. E. Savage, Ind. Eng. Chem. Res., 2015, 54, 4066–4071 CrossRef CAS.
- R. B. Levine, C. O. S. Sierra, R. Hockstad, W. Obeid, P. G. Hatcher and P. E. Savage, Environ. Prog. Sustainable Energy, 2013, 32, 962–975 CrossRef CAS.
- X. Cheng, J. Riordon, B. Nguyen, M. D. Ooms and D. Sinton, Green Chem., 2017, 106–111 RSC.
- R. Praveenkumar, K. Lee, J. Lee and Y.-K. Oh, Green Chem., 2015, 17, 1226–1234 RSC.
- R. K. Desai, M. Streefland, R. H. Wijffels and M. H. M. Eppink, Green Chem., 2016, 18, 1261–1267 RSC.
- H. Taher, S. Al-Zuhair, A. H. Al-Marzouqi, Y. Haik and M. Farid, Biomass Bioenergy, 2014, 66, 159–167 CrossRef CAS.
- J. Cheng, R. Huang, T. Li, J. Zhou and K. Cen, Bioresour. Technol., 2014, 170, 69–75 CrossRef CAS PubMed.
- C. Dejoye Tanzi, M. Abert Vian and F. Chemat, Bioresour. Technol., 2013, 134, 271–275 CrossRef CAS PubMed.
- Y.-X. Huo, K. M. Cho, J. G. L. Rivera, E. Monte, C. R. Shen, Y. Yan and J. C. Liao, Nat. Biotechnol., 2011, 29, 346–351 CrossRef CAS PubMed.
- C. Li and H. H. P. Fang, Crit. Rev. Environ. Sci. Technol., 2007, 37, 1–39 CrossRef CAS.
- A. J. Ward, D. M. Lewis and F. B. Green, Algal Res., 2014, 5, 204–214 CrossRef.
- A.-M. Lakaniemi, O. H. Tuovinen and J. A. Puhakka, Bioresour. Technol., 2013, 135, 222–231 CrossRef CAS PubMed.
- K. Chidambarampadmavathy, O. P. Karthikeyan and K. Heimann, Eng. Life Sci., 2015, 15, 689–699 CrossRef CAS.
- C. Zamalloa, E. Vulsteke, J. Albrecht and W. Verstraete, Bioresour. Technol., 2011, 102, 1149–1158 CrossRef CAS PubMed.
- D. Klemm, B. Heublein, H.-P. Fink and A. Bohn, Angew. Chem., Int. Ed., 2005, 44, 3358–3393 CrossRef CAS PubMed.
- S. Heaven, J. Milledge and Y. Zhang, Biotechnol. Adv., 2011, 29, 164–167 CrossRef CAS PubMed.
- D. Levin, Int. J. Hydrogen Energy, 2004, 29, 173–185 CrossRef CAS.
- Y. Ghasemi, S. Rasoul-Amini, A. T. Naseri, N. Montazeri-Najafabady, M. A. Mobasher and F. Dabbagh, Appl. Biochem. Microbiol., 2012, 48, 126–144 CrossRef CAS.
- J. R. Benemann, J. Appl. Phycol., 2000, 12, 291–300 CrossRef CAS.
- M. Daroch, S. Geng and G. Wang, Appl. Energy, 2013, 102, 1371–1381 CrossRef.
- R. Radakovits, R. E. Jinkerson, A. Darzins and M. C. Posewitz, Eukaryotic Cell, 2010, 9, 486–501 CrossRef CAS PubMed.
- R. H. Wijffels, O. Kruse and K. J. Hellingwerf, Curr. Opin. Biotechnol., 2013, 24, 405–413 CrossRef CAS PubMed.
- H. Gaffron and J. Rubin, J. Gen. Physiol., 1942, 26, 219–240 CrossRef CAS PubMed.
- K. Kumar and D. Das, in Natural and Artificial Photosynthesis, John Wiley & Sons Inc., Hoboken, NJ, USA, 2013, pp. 173–215 Search PubMed.
- D.-J. Lee, J.-S. Chang and J.-Y. Lai, Bioresour. Technol., 2015, 198, 891–895 CrossRef CAS PubMed.
- P. Bombelli, M. Zarrouati, R. J. Thorne, K. Schneider, S. J. L. Rowden, A. Ali, K. Yunus, P. J. Cameron, A. C. Fisher, D. Ian Wilson, C. J. Howe and A. J. McCormick, Phys. Chem. Chem. Phys., 2012, 14, 12221 RSC.
- D. J. Lea-Smith, N. Ross, M. Zori, D. S. Bendall, J. S. Dennis, S. a. Scott, A. G. Smith and C. J. Howe, Plant Physiol., 2013, 162, 484–495 CrossRef CAS PubMed.
- R. W. Bradley, P. Bombelli, D. J. Lea-Smith and C. J. Howe, Phys. Chem. Chem. Phys., 2013, 15, 13611 RSC.
- P. Bombelli, D. M. R. Iyer, S. Covshoff, A. J. McCormick, K. Yunus, J. M. Hibberd, A. C. Fisher and C. J. Howe, Appl. Microbiol. Biotechnol., 2013, 97, 429–438 CrossRef CAS PubMed.
- A. J. McCormick, P. Bombelli, R. W. Bradley, R. Thorne, T. Wenzel and C. J. Howe, Energy Environ. Sci., 2015, 8, 1092–1109 CAS.
- J. L. White, M. F. Baruch, J. E. Pander, Y. Hu, I. C. Fortmeyer, J. E. Park, T. Zhang, K. Liao, J. Gu, Y. Yan, T. W. Shaw, E. Abelev and A. B. Bocarsly, Chem. Rev., 2015, 115, 12888–12935 CrossRef CAS PubMed.
- J. Qiao, Y. Liu, F. Hong and J. Zhang, Chem. Soc. Rev., 2014, 43, 631–675 RSC.
Footnote |
| † Equally contributing author. |
| This journal is © The Royal Society of Chemistry 2018 |