Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Donor–acceptor interactions between cyclic trinuclear pyridinate gold(I)-complexes and electron-poor guests: nature and energetics of guest-binding and templating on graphite†
Raiko
Hahn
a,
Fabian
Bohle
b,
Stefan
Kotte
c,
Tristan J.
Keller
c,
Stefan-S.
Jester
 c,
Andreas
Hansen
c,
Andreas
Hansen
 b,
Stefan
Grimme
b,
Stefan
Grimme
 b and
Birgit
Esser
b and
Birgit
Esser
 *a
*a
aInstitute for Organic Chemistry, University of Freiburg, Albertstraße 21, 79104 Freiburg, Germany. E-mail: besser@oc.uni-freiburg.de
bMulliken Center for Theoretical Chemistry, University of Bonn, Beringstraße 4, 53115 Bonn, Germany
cKekulé Institute for Organic Chemistry and Biochemistry, University of Bonn, Gerhard-Domagk-Straße 1, 53121 Bonn, Germany
First published on 5th March 2018
Abstract
Donor–acceptor-type interactions between π-electron systems are of high relevance in the design of chemical sensors. Due to their electron-rich nature, cyclic trinuclear complexes (CTCs) of gold(I) are ideal receptor sites for electron-deficient aromatic analytes. Scanning tunneling microscopy provided insight into the structures of two-dimensional crystals of pyridinate gold CTCs that form on a graphite template at the solid/liquid interface. One polymorph thereof – in turn – templated the on-top co-adsorption of π-acidic pyrazolate CTCs as electron-poor guests up to a certain threshold. From NMR titration experiments, we quantified free energies of −6.1 to −7.5 kcal mol−1 for the binding between pyridinate gold(I) CTCs and π-acidic pyrazolate CTCs. Quantum chemical calculations revealed that these interactions are largely dominated by London dispersion. These results give a more detailed insight into a rational design of sensitive CNT- or graphene-based sensors for π-acidic analytes, such as electron-deficient aromatics.
Introduction
Non-covalent interactions between π–systems and cations, anions or aromatics are found in biological systems and are important in, among others, host/guest chemistry, self-assembly,1–3 and molecular machines.4–7 Donor–acceptor-type interactions between planar arenes8 also play an important role in the design of chemical sensors, for instance for the detection of electron-poor nitroaromatics, such as 1,3,5-trinitrotoluene, TNT, or structurally related explosives.9 The field of resistivity-based sensing – using carbon nanotubes (CNTs) or graphene – is emerging due to the operational simplicity of such devices, their small size and potential low cost.10,11 In a CNT- or graphene-based sensor, however, binding of the analyte to the carbon surface is unspecific and often of insufficient strength.11–13 Aiming at the detection of electron-deficient aromatics, functionalization of the surface with a complementary electron-rich π-system could selectively template and increase the binding strength to a specific analyte compound or a group thereof. Non-covalent doping of the carbon-based material by adsorption of a monolayer-forming species is proposed to lead to changes in the electronic band structure. This may affect the electrical resistivity in a sensing setup – without requiring a covalent doping of the carbon material itself, which may introduce defects or disorder.14Due to their planar, cyclic structure and the electron-rich nature of their π-system, cyclic trinuclear complexes (CTCs) of gold(I)15–17 with pyridinate ligands (1, Fig. 1a) are promising candidates for the design of such graphene-based sensors. Our use of pyridine ligands with long alkyl substituents aimed at a two-dimensional (2D) supramolecular engineering approach towards packing of oblate CTCs on planar carbon-based materials, such as graphite or graphene.18 Au(I) CTCs have been shown to possess a high electron-density above and below the trinuclear gold core, also referred to as high π-basicity.19 Modification of a 2D carbon surface with complexes 1 could increase surface binding to electron-poor analytes, as depicted in Fig. 1b. The selective binding of an analyte on top of the active sites of the monolayer would effectively modulate charge transfer to the carbon surface, thereby leading to a change in electrical conductivity.
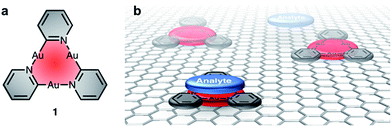 | ||
| Fig. 1 (a) Electron-rich cyclic trinuclear pyridinate gold(I) complexes 1 and (b) concept of functionalizing a CNT or graphene surface with 1 to template the binding of electron-poor analytes. | ||
Scanning tunneling microscopy (STM) is a tool to visualize receptor-analyte adducts that form or deposit on a solid surface. We used highly-oriented pyrolytic graphite (HOPG) as the bulk analogue of graphene and as model system for adsorption experiments by STM. Au(I)-CTCs have previously been investigated by this method after adsorption from various solvents.20 In addition, long alkyl/alkoxy side-chains are known to separate large macrocyclic backbones and determine the adsorption/packing into 2D crystalline supramolecular nanopatterns on graphite,21 a strategy that has previously been used for the design and high-resolution imaging of Au(I)-CTCs.22 A particular aim was to investigate whether monolayers of CTCs of type 1 could template the co-adsorption of electron-poor guests, which may be important for future sensing applications. To validate our concept, we chose electron-deficient guests with a known high π-acidity, namely pyrazolate CTCs 2a–c (Fig. 2).19,23–26 While donor–acceptor-type interactions with electron-poor π-systems have not yet been reported for pyridinate CTCs of type 1, they have been observed for imidazolate or carbeniate CTCs.16,27–33 However, attempts to quantify these interactions have not been reported up to date, and no account exists to study these interactions at the solid–liquid interface. We herein present a study on donor–acceptor-type interactions of pyridinate CTCs of type 1 with π-acidic guests 2. We provide interaction free energies (determined through NMR-investigations and quantum chemical calculations for these compounds in solution) and visualize self-assembled monolayers of 1e as well as co-adsorbates of 1e and 2c by STM at the solid/liquid interface. These results may aid the design of sensitive and selective sensors, where the graphite surface functionalized with CTCs 1 used in STM measurements is a model system for a chemoresistive CNT- or graphene-based sensor.
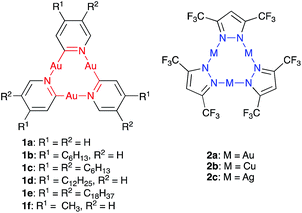 | ||
| Fig. 2 Electron-rich cyclic trinuclear pyridinate gold(I) complexes 1a–f and electron-poor pyrazolate complexes 2a–c used in this study. | ||
Results and discussion
Synthesis of pyridinate CTCs 1
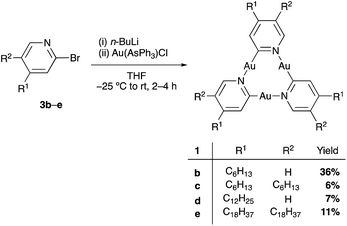
The pyridinate complex 1a was the first CTC to be described by Vaughan in 1970,34 and its structure was confirmed through X-ray crystallography 30 years later by Balch and coworkers.35 In order to obtain derivatives of CTCs 1 with the ability to form stable 2D self-assemblies on HOPG for STM studies and with high solubility in organic solvents for NMR titration experiments, one (1b, d) or two (1c, e) alkyl chains of different lengths were attached to each pyridine ring.Starting from the 2-bromo pyridines 3b–e (for synthesis see ESI†), the synthesis of CTCs 1b–e was accomplished using a modification of Vaughan's procedure (Scheme 1).34 Several column chromatography and recrystallization steps led to pure samples of 1b, d, and e. Pyridinate CTC 1c with six hexyl chains, however, could not be obtained in pure form (see ESI†). 1b and 1d showed excellent solubility in common organic solvents, and 1e became soluble at temperatures above 40 °C. This allowed for their characterization by NMR spectroscopy and MALDI-TOF analysis. All synthesized pyridinate CTCs 1 were somewhat light-sensitive but did not decompose in high vacuum. Compared to the unsubstituted derivative 1a,341b, 1d, and 1e were less prone to decomposition in solution and could be handled for a prolonged amount of time, likely due to the shielding effect of the alkyl chains on the trinuclear gold centers. Pyrazolate CTCs 2a–c were synthesized according to literature procedures23,36 and were obtained in excellent purity. They showed high solubility in benzene, but were somewhat light-sensitive.
Determination of binding constants between CTCs 1 and 2a–c
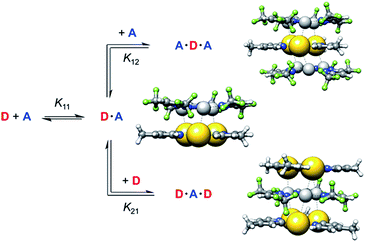
The binding constants between the pyridinate CTCs 1b and 1d as π-donors (D) and electron-deficient CTCs 2a–c as π-acceptors (A) were determined using 1H NMR titration experiments.37 UV/vis spectra of mixtures of 1d and acceptors 2a–c showed the appearance of a small charge-transfer band between 250 and 350 nm (see ESI†). However, due to significant overlap of the absorption maxima of the two compounds UV/vis spectroscopy was not employed to determine binding constants.Compared to common host/guest systems, a quantitative analysis of π–π interactions in the case of CTCs is more difficult to achieve, since due to the planarity of the molecules both the donor and the acceptor possess two equal binding sites. Hence not only the formation of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, but also of 2
1, but also of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complexes and higher order structures is possible (Scheme 2). In the NMR titrations reported herein, the acceptor was added to the donor, and in most data points collected the acceptor was present in excess. Hence the formation of 1
2 complexes and higher order structures is possible (Scheme 2). In the NMR titrations reported herein, the acceptor was added to the donor, and in most data points collected the acceptor was present in excess. Hence the formation of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (D·A) and 1
1 (D·A) and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (A·D·A) complexes was considered, while 2
2 (A·D·A) complexes was considered, while 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (D·A·D) complexes were neglected. The results of the titration experiments were fitted using non-linear regression to a 1
1 (D·A·D) complexes were neglected. The results of the titration experiments were fitted using non-linear regression to a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (D
2 (D![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A) binding model,37 employing an online tool provided by Thordarson and coworkers.38,39 All measurements showed a fast exchange between donor and acceptor in relation to the NMR time scale, resulting in one set of signals for the donor and for the acceptor. Selected 1H NMR spectra from a titration of 1b with 2a are shown in Fig. 3. Titration experiments of CTC 1b with the three acceptors 2a–c, performed three times at slightly different concentrations (see ESI†), yielded the binding constants and free energies of binding listed in Table 1. These values confirm that the π-acidity of the pyrazolate CTCs 2 increases in the order Au < Cu < Ag, as predicted by Omary et al.23
A) binding model,37 employing an online tool provided by Thordarson and coworkers.38,39 All measurements showed a fast exchange between donor and acceptor in relation to the NMR time scale, resulting in one set of signals for the donor and for the acceptor. Selected 1H NMR spectra from a titration of 1b with 2a are shown in Fig. 3. Titration experiments of CTC 1b with the three acceptors 2a–c, performed three times at slightly different concentrations (see ESI†), yielded the binding constants and free energies of binding listed in Table 1. These values confirm that the π-acidity of the pyrazolate CTCs 2 increases in the order Au < Cu < Ag, as predicted by Omary et al.23
 | ||
| Scheme 2 Equilibria of complex formation between gold pyridinate CTC 1f as donor D and silver pyrazolate 2c as acceptor A (PBEh-3c calculated structures). | ||
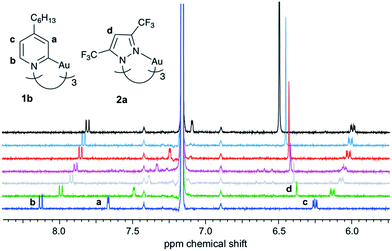 | ||
| Fig. 3 1H NMR titration of 1b with 2a (C6D6, 300 K; added equivalents of 2a from bottom to top: 0, 0.6, 1.1, 1.5, 2.5, 4.0, 11). | ||
The binding constants K11 for the formation of D·A complexes lie between 3 × 105 and 3 × 104 L mol−1, corresponding to free binding energies ΔG11 between −6.1 and −7.5 kcal mol−1. These values are in the typical range of binding free energies for common supramolecular complexes.6,8,40 For comparison, the mean of all experimental free energies of the S30L41 (benchmark set of realistic host-guest complexes) amounts to −6.5 kcal mol−1. This demonstrates that pyridinate CTCs 1 are promising candidates to strongly bind electron-poor analytes.
Due to a lowering of the electron density of the donor in the D·A complex, the binding constants K12 for the formation of complexes A·D·A are significantly smaller than K11. This indicates an anti-cooperative binding, since a ratio of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 between K11 and K12 would be expected in the statistical case.37
1 between K11 and K12 would be expected in the statistical case.37
All binding isotherms were also fitted to a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 model, which clearly showed its inferiority over the 1
1 model, which clearly showed its inferiority over the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 model. The deviations between experimental and fitted values (so-called residuals) were abound ten times higher than for the 1
2 model. The deviations between experimental and fitted values (so-called residuals) were abound ten times higher than for the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 model and showed sinusoidal shape (see ESI†).42 Binding models corresponding to higher-order structures were not employed, since K12 was already found to be two or three orders of magnitude smaller than K11.
2 model and showed sinusoidal shape (see ESI†).42 Binding models corresponding to higher-order structures were not employed, since K12 was already found to be two or three orders of magnitude smaller than K11.
A comparison with the binding constants determined for acceptor 2c with donor 1d, carrying three dodecyl groups, of K11 = 2.81 × 105 L mol−1 and K12 = 9440 L mol−1 showed that the length of the alkyl groups on 1 did not affect its binding ability. This was important with respect to the STM measurements described below, where pyridinate complex 1e with octadecyl groups was used.
Quantum chemical calculations, employing an established multilevel Ansatz,40,43 provided association free energies for the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes of 1b with 2a–c (Table 2). DFT calculations on gold CTCs have been performed before, however, with the intention to investigate their self-assembly on HOPG.20 For computational efficiency the alkyl side chains of donor 1b were replaced by methyl groups (1f, Fig. 2). Geometries were optimized with the DFT composite scheme PBEh-3c,44 and energies (ΔE) were refined with PBE0-D345,46 as a dispersion-corrected hybrid functional using the large def2-QZVP-f/-g47 basis set. Thermostatistical contributions to free energies ΔGRRHO were evaluated at the PBEh-3c level of theory. COSMO-RS48 provided solvation free energies (ΔδGsolv) in a black-box fashion.
1 complexes of 1b with 2a–c (Table 2). DFT calculations on gold CTCs have been performed before, however, with the intention to investigate their self-assembly on HOPG.20 For computational efficiency the alkyl side chains of donor 1b were replaced by methyl groups (1f, Fig. 2). Geometries were optimized with the DFT composite scheme PBEh-3c,44 and energies (ΔE) were refined with PBE0-D345,46 as a dispersion-corrected hybrid functional using the large def2-QZVP-f/-g47 basis set. Thermostatistical contributions to free energies ΔGRRHO were evaluated at the PBEh-3c level of theory. COSMO-RS48 provided solvation free energies (ΔδGsolv) in a black-box fashion.
| Acceptor | ΔE (PBE0-D3/QZ-f/-g) | ΔGRRHO (PBEh-3c) | ΔδGsolv | ΔGcalc 11 |
|---|---|---|---|---|
| a All values are given in kcal mol−1. b Obtained as the sum of electronic interaction energies ΔE, thermostatistical contributions ΔGRRHO and solvation free energies ΔδGsolv. | ||||
| 2a (M = Au) | −32.1 | 17.4 | 4.6 | −10.1 |
| 2b (M = Cu) | −38.0 | 17.5 | 6.1 | −14.3 |
| 2c (M = Ag) | −39.5 | 17.3 | 6.0 | −16.3 |
For the binding free energies between pyridinate gold CTC 1f and pyrazolate CTCs 2a–c, a reasonable agreement of (shifted) theoretical and experimental ΔGcalc 11 values was observed. The experimental trend of increasing acceptor strength in going from 2a to 2c was clearly reproduced. These stacked CTCs are to a large extent stabilized by London dispersion. For the complex of 1f·2c (without alkyl side chains) the dispersion interaction energy (D3(PBE0) level) amounted to −31.3 kcal mol−1, which accounts for 79% of the total interaction energy ΔE. The same trend was obtained with our recently developed D4 dispersion model,49 which additionally takes into account the influence of the electronic structure on the dynamic polarizability and thus the dispersion energy (see ESI†).
To further validate the DFT-calculated interaction energies, a high-level coupled-cluster value employing DLPNO-CCSD(T)50,51/TightPNO52/CBS53(def2-TZVPP47/def2-QZVPP54) for the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex between 1f and 2c was calculated, yielding an electronic gas phase interaction energy of ΔE11 = −39.2 (±3) kcal mol−1. This value is in very good agreement with the corresponding PBE0-D3/def2-QZVP-f/-g result of −39.5 kcal mol−1, including 6.8 kcal mol−1 deformation energy mostly originating from the geometry relaxation of 2c. Tentatively, the deviation to the experiment can be attributed to the neglect of the alkyl side chains on the donor 1f and/or the implicit solvation model, which may be improperly parameterized for coinage metal cations within these planar structures.55
1 complex between 1f and 2c was calculated, yielding an electronic gas phase interaction energy of ΔE11 = −39.2 (±3) kcal mol−1. This value is in very good agreement with the corresponding PBE0-D3/def2-QZVP-f/-g result of −39.5 kcal mol−1, including 6.8 kcal mol−1 deformation energy mostly originating from the geometry relaxation of 2c. Tentatively, the deviation to the experiment can be attributed to the neglect of the alkyl side chains on the donor 1f and/or the implicit solvation model, which may be improperly parameterized for coinage metal cations within these planar structures.55
Investigation of donor–acceptor-type interactions using STM
Finally, the interaction between a pyridinate gold CTC of type 1 and a pyrazolate acceptor of type 2 was investigated at the solid/liquid interface using STM. The HOPG surface is an excellent model system for graphene-based systems, such as in the proposed sensors. Our study aimed at elucidating whether this surface could be functionalized with electron-rich CTCs 1. Up to date only two reports of STM measurements on CTCs have been published on pyrazolate and carbeniate complexes on HOPG.20,22Concentration-20,22 and solvent-dependent56 polymorphisms are well-known phenomena.
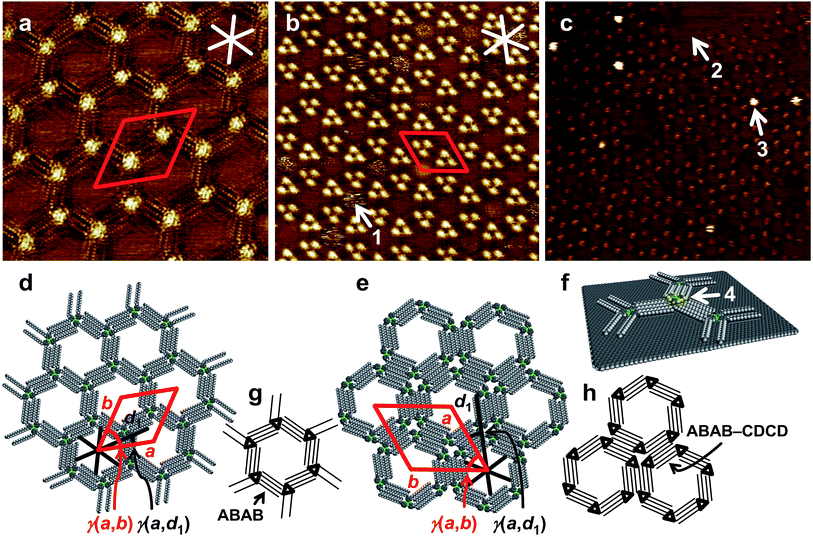
Here, at an increased concentration for 1e of 10−5 M, the molecules assembled in the more densely packed polymorph B (Fig. 4b and e). Two out of six alkyl chains of each molecule were not adsorbed on the HOPG surface but pointed towards the solution phase. Hexagonal assemblies resulted, likely with the same chiral packing as described in Fig. 4d and g, but with a counterclockwise orientation (Fig. 4h). Each corner of the hexagonal units appeared as a bright spot, representing the molecular backbones. The hexagonal assemblies packed densely to form an – again – hexagonal pattern (Fig. 4e and h), to which a unit cell of a = b = (7.1 ± 0.2) nm, γ(a,b) = (60 ± 2)° was indexed. The unit cell vector a was oriented at an angle γ(a,d1) = (19 ± 2)° relative to the HOPG main axis direction d1. Each (indexed) unit cell contained six molecules of 1e. As a result of the hierarchical packing of hexagonal units (each formed by six molecules), triples of bright spots, containing three neighboring backbones, were observed. The bright regions in the interior of some of the pores (indicated by arrow 1 in Fig. 4b) appeared blurred, which was attributed to unspecifically adsorbed molecules that most probably moved/rotated faster than the timescale of the measurement. Similar studies on pore filling have been performed before with pyrene as guest molecule20 and have also been extended to larger guest molecules.57 Even at this increased concentration, no evidence for the formation of multilayers, where molecules of 1e stacked on top of each other, was observed in any of the images acquired. This absence of multilayer stacks of 1e on HOPG even at increased concentrations was highly relevant for being able to correctly conclude – from STM topography data – a possible formation/adsorption of mixed stacks.
This result shows that gold pyridinate CTCs 1 are promising molecules in the design of CNT- or graphene-based sensors for electron-poor aromatics, where surface-functionalization with 1 would increase binding of the analyte. Furthermore, to the best of our knowledge, this is the first report of a co-adsorption, studied by STM, which is directed by a donor–acceptor-type interaction between a π-basic and a π-acidic molecule. Several studies have been published, where the pores of self-assembled 2D-networks on HOPG were filled with guest molecules58–64 or where the adlayer of one macrocycle was used as a template for another macrocycle.65 However, donor–acceptor-type interactions in the context of the formation of a double layer at the solid/liquid interface have only been discussed for STM-observations of the co-deposition of C60 with thiophene-containing macrocycles.66,67 The results discussed herein will stimulate us to (in the future) enhance the π-basicity of donor compounds on HOPG and to quantify host-guest binding on the surface.
Conclusions
In conclusion, we have shown through NMR titration experiments that the free energies of binding between gold(I) pyridinate CTCs 1 and coinage-metal pyrazolate CTCs 2 lie between −6.1 and −7.5 kcal mol−1. This places them in the typical range compared to common supramolecular host-guest complexes, where the host possesses a cavity. High-level quantum chemical calculations revealed that these interactions are largely stabilized by London dispersion. Their strongly π-basic nature makes pyridinate CTCs 1 attractive as binding sites for electron-poor molecules, for instance in the design of chemical sensors. We demonstrated this concept by using pyridinate CTC 1e to template the co-adsorption of silver(I) pyrazolate CTC 2c on HOPG. These results give a more detailed insight into a rational design of sensitive CNT- or graphene-based sensors for π-acidic analytes, such as electron-deficient aromatics, with potential for a better sensitivity for π-acidic analytes as compared to existing detection systems.Conflicts of interest
There are no conflicts to declare.Acknowledgements
R. H. thanks G. Studer, T. Lechtenberg and L. Huber for preparative assistance, M. Engeser for measurement of MALDI spectra, S. J. thanks M. Plümper for STM imaging, supramolecular modelling and discussions, and B. E. thanks D. den Boer for helpful discussions. Financial support through the Jürgen Manchot Stiftung (graduate fellowship for R. H.) and the DFG (Emmy Noether grant ES 361/2-1 for B. E., Priority Program SPP 1807, “Control of Dispersion Interactions in Chemistry”) is gratefully acknowledged.References
- Z. Chen, A. Lohr, C. R. Saha-Moller and F. Würthner, Chem. Soc. Rev., 2009, 38, 564–584 RSC.
- F. J. M. Hoeben, P. Jonkheijm, E. W. Meijer and A. P. H. J. Schenning, Chem. Rev., 2005, 105, 1491–1546 CrossRef CAS PubMed.
- A. Das and S. Ghosh, Angew. Chem., Int. Ed., 2014, 53, 2038–2054 CrossRef CAS PubMed.
- S. Erbas-Cakmak, D. A. Leigh, C. T. McTernan and A. L. Nussbaumer, Chem. Rev., 2015, 115, 10081–10206 CrossRef CAS PubMed.
- K. Ariga and T. Kunitake, Supramolecular Chemistry – Fundamentals and Applications, Springer-Verlag, Berlin, Heidelberg, 2006 Search PubMed.
- J. W. Steed and J. L. Atwood, Supramolecular Chemistry, John Wiley & Sons, Ltd., Chichester, West Sussex, UK, 2009 Search PubMed.
- J.-M. Lehn, Supramolecular Chemistry, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, FRG, 1995 Search PubMed.
- R. Gleiter and G. Haberhauer, in Aromaticity and Other Conjugation Effects, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2012, pp. 181–216 Search PubMed.
- J.-S. Yang and T. M. Swager, J. Am. Chem. Soc., 1998, 120, 5321–5322 CrossRef CAS.
- K. A. Mirica, J. G. Weis, J. M. Schnorr, B. Esser and T. M. Swager, Angew. Chem., Int. Ed., 2012, 51, 10740–10745 CrossRef CAS PubMed.
- J. F. Fennell, S. F. Liu, J. M. Azzarelli, J. G. Weis, S. Rochat, K. A. Mirica, J. B. Ravnsbaek and T. M. Swager, Angew. Chem., Int. Ed., 2016, 55, 1266–1281 CrossRef CAS PubMed.
- D. R. Kauffman and A. Star, Angew. Chem., Int. Ed., 2008, 47, 6550–6570 CrossRef CAS PubMed.
- E. S. Snow, F. K. Perkins and J. A. Robinson, Chem. Soc. Rev., 2006, 35, 790 RSC.
- B. Li, A. V. Klekachev, M. Cantoro, C. Huyghebaert, A. Stesmans, I. Asselberghs, S. De Gendt and S. De Feyter, Nanoscale, 2013, 5, 9640 RSC.
- Gold Chemistry – Applications and Future Directions in the Life Sciences, ed. F. Mohr, Wiley-VCH, 2009 Search PubMed.
- A. Burini, A. A. Mohamed and J. P. Fackler, Comments Inorg. Chem., 2003, 24, 253–280 CrossRef CAS.
- H. E. Abdou, A. A. Mohamed, J. P. Fackler, A. Burini, R. Galassi, J. M. López-de-Luzuriaga and M. E. Olmos, Coord. Chem. Rev., 2009, 253, 1661–1669 CrossRef CAS.
- A. Ciesielski, S. Colella, L. Zalewski, B. Bruchmann and P. Samorì, CrystEngComm, 2011, 13, 5535 RSC.
- S. M. Tekarli, T. R. Cundari and M. A. Omary, J. Am. Chem. Soc., 2008, 130, 1669–1675 CrossRef CAS PubMed.
- B. Chilukuri, R. N. McDougald, M. M. Ghimire, V. N. Nesterov, U. Mazur, M. A. Omary and K. W. Hipps, J. Phys. Chem. C, 2015, 119, 24844–24858 CAS.
- S.-S. Jester, E. Sigmund and S. Höger, J. Am. Chem. Soc., 2011, 133, 11062–11065 CrossRef CAS PubMed.
- D. den Boer, M. Krikorian, B. Esser and T. M. Swager, J. Phys. Chem. C, 2013, 117, 8290–8298 CAS.
- M. A. Omary, M. A. Rawashdeh-Omary, M. W. A. Gonser, O. Elbjeirami, T. Grimes, T. R. Cundari, H. V. K. Diyabalanage, C. S. P. Gamage and H. V. R. Dias, Inorg. Chem., 2005, 44, 8200–8210 CrossRef CAS PubMed.
- H. V. R. Dias, C. S. P. Gamage, J. Keltner, H. V. K. Diyabalanage, I. Omari, Y. Eyobo, N. R. Dias, N. Roehr, L. McKinney and T. Poth, Inorg. Chem., 2007, 46, 2979–2987 CrossRef CAS PubMed.
- H. V. Rasika Dias and C. S. P. Gamage, Angew. Chem., Int. Ed., 2007, 46, 2192–2194 CrossRef PubMed.
- M. a Omary, O. Elbjeirami, C. S. P. Gamage, K. M. Sherman and H. V. R. Dias, Inorg. Chem., 2009, 48, 1784–1786 CrossRef CAS PubMed.
- A. Burini, J. P. Fackler, R. Galassi, T. A. Grant, M. A. Omary, M. A. Rawashdeh-Omary, B. R. Pietroni and R. J. Staples, J. Am. Chem. Soc., 2000, 122, 11264–11265 CrossRef CAS.
- M. M. Olmstead, F. Jiang, S. Attar and A. L. Balch, J. Am. Chem. Soc., 2001, 123, 3260–3267 CrossRef CAS PubMed.
- M. A. Rawashdeh-Omary, M. A. Omary, J. P. Fackler, R. Galassi, B. R. Pietroni and A. Burini, J. Am. Chem. Soc., 2001, 123, 9689–9691 CrossRef CAS PubMed.
- A. Burini, J. P. Fackler, R. Galassi, A. Macchioni, M. A. Omary, M. A. Rawashdeh-Omary, B. R. Pietroni, S. Sabatini and C. Zuccaccia, J. Am. Chem. Soc., 2002, 124, 4570–4571 CrossRef CAS PubMed.
- A. A. Mohamed, M. A. Rawashdeh-Omary, M. A. Omary and J. P. Fackler Jr, Dalton Trans., 2005, 2597 RSC.
- M. A. Omary, A. A. Mohamed, M. A. Rawashdeh-Omary and J. P. Fackler, Coord. Chem. Rev., 2005, 249, 1372–1381 CrossRef CAS.
- O. Elbjeirami, M. D. Rashdan, V. Nesterov and M. A. Rawashdeh-Omary, Dalton Trans., 2010, 39, 9465 RSC.
- L. G. Vaughan, J. Am. Chem. Soc., 1970, 92, 730–731 CrossRef CAS.
- A. Hayashi, M. M. Olmstead, S. Attar and A. L. Balch, J. Am. Chem. Soc., 2002, 124, 5791–5795 CrossRef CAS PubMed.
- H. Rasika Dias, S. A. Polach and Z. Wang, J. Fluorine Chem., 2000, 103, 163–169 CrossRef.
- P. Thordarson, Chem. Soc. Rev., 2011, 40, 1305–1323 RSC.
- D. Brynn Hibbert and P. Thordarson, Chem. Commun., 2016, 52, 12792–12805 RSC.
- htttp://www.supramolecular.org .
- S. Grimme, Chem.–Eur. J., 2012, 18, 9955–9964 CrossRef CAS PubMed.
- R. Sure and S. Grimme, J. Chem. Theory Comput., 2015, 11, 3785–3801 CrossRef CAS PubMed.
- F. Ulatowski, K. Dabrowa, T. Bałakier and J. Jurczak, J. Org. Chem., 2016, 81, 1746–1756 CrossRef CAS PubMed.
- D. Zell, M. Bursch, V. Müller, S. Grimme and L. Ackermann, Angew. Chem., Int. Ed., 2017, 56, 10378–10382 CrossRef CAS PubMed.
- S. Grimme, J. G. Brandenburg, C. Bannwarth and A. Hansen, J. Chem. Phys., 2015, 143, 54107 CrossRef PubMed.
- C. Adamo and V. Barone, J. Chem. Phys., 1999, 110, 6158–6170 CrossRef CAS.
- M. Ernzerhof and G. E. Scuseria, J. Chem. Phys., 1999, 110, 5029–5036 CrossRef CAS.
- F. Weigend and R. Ahlrichs, Phys. Chem. Chem. Phys., 2005, 7, 3297–3305 RSC.
- A. Klamt, J. Phys. Chem., 1995, 99, 2224–2235 CrossRef CAS.
- E. Caldeweyher, C. Bannwarth and S. Grimme, J. Chem. Phys., 2017, 147, 34112 CrossRef PubMed.
- C. Riplinger, B. Sandhoefer, A. Hansen and F. Neese, J. Chem. Phys., 2013, 139, 134101 CrossRef PubMed.
- C. Riplinger, P. Pinski, U. Becker, E. F. Valeev and F. Neese, J. Chem. Phys., 2016, 144, 24109 CrossRef PubMed.
- F. Pavošević, C. Peng, P. Pinski, C. Riplinger, F. Neese and E. F. Valeev, J. Chem. Phys., 2017, 146, 174108 CrossRef PubMed.
- F. Neese and E. F. Valeev, J. Chem. Theory Comput., 2011, 7, 33–43 CrossRef CAS PubMed.
- A. Hellweg, C. Hättig, S. Höfener and W. Klopper, Theor. Chem. Acc., 2007, 117, 587–597 CrossRef CAS.
- J. Antony, R. Sure and S. Grimme, Chem. Commun., 2015, 51, 1764–1774 RSC.
- K. Tahara, S. Furukawa, H. Uji-i, T. Uchino, T. Ichikawa, J. Zhang, W. Mamdouh, M. Sonoda, F. C. De Schryver, S. De Feyter and Y. Tobe, J. Am. Chem. Soc., 2006, 128, 16613–16625 CrossRef CAS PubMed.
- J. Adisoejoso, K. Tahara, S. Okuhata, S. Lei, Y. Tobe and S. De Feyter, Angew. Chem., Int. Ed., 2009, 48, 7353–7357 CrossRef CAS PubMed.
- S. Furukawa, K. Tahara, F. C. De Schryver, M. Van der Auweraer, Y. Tobe and S. De Feyter, Angew. Chem., Int. Ed., 2007, 46, 2831–2834 CrossRef CAS PubMed.
- S. Lei, K. Tahara, X. Feng, S. Furukawa, F. C. De Schryver, K. Müllen, Y. Tobe and S. De Feyter, J. Am. Chem. Soc., 2008, 130, 7119–7129 CrossRef CAS PubMed.
- K. Tahara, S. Lei, W. Mamdouh, Y. Yamaguchi, T. Ichikawa, H. Uji-i, M. Sonoda, K. Hirose, F. C. De Schryver, S. De Feyter and Y. Tobe, J. Am. Chem. Soc., 2008, 130, 6666–6667 CrossRef CAS PubMed.
- K. Tahara, S. Lei, D. Mössinger, H. Kozuma, K. Inukai, M. Van der Auweraer, F. C. De Schryver, S. Höger, Y. Tobe and S. De Feyter, Chem. Commun., 2008, 3897–3899 RSC.
- B. Schmaltz, A. Rouhanipour, H. J. Räder, W. Pisula and K. Müllen, Angew. Chem., Int. Ed., 2009, 48, 720–724 CrossRef CAS PubMed.
- H. Shimizu, J. D. Cojal González, M. Hasegawa, T. Nishinaga, T. Haque, M. Takase, H. Otani, J. P. Rabe and M. Iyoda, J. Am. Chem. Soc., 2015, 137, 3877–3885 CrossRef CAS PubMed.
- J. Teyssandier, S. De Feyter and K. S. Mali, Chem. Commun., 2016, 52, 11465–11487 RSC.
- T. Chen, G.-B. Pan, H. Wettach, M. Fritzsche, S. Höger, L.-J. Wan, H.-B. Yang, B. H. Northrop and P. J. Stang, J. Am. Chem. Soc., 2010, 132, 1328–1333 CrossRef CAS PubMed.
- G.-B. Pan, X.-H. Cheng, S. Höger and W. Freyland, J. Am. Chem. Soc., 2006, 128, 4218–4219 CrossRef CAS PubMed.
- E. Mena-Osteritz and P. Bäuerle, Adv. Mater., 2006, 18, 447–451 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic manipulations, details on NMR titration experiments, stability study on 1b, NMR and UV/vis absorption spectra, details on quantum chemical calculations and STM measurements. See DOI: 10.1039/c7sc05355j |
| This journal is © The Royal Society of Chemistry 2018 |