Open Access Article
Open Access ArticleEnhancement of CO2 binding and mechanical properties upon diamine functionalization of M2(dobpdc) metal–organic frameworks†
Jung-Hoon
Lee
 ab,
Rebecca L.
Siegelman
ab,
Rebecca L.
Siegelman
 cd,
Lorenzo
Maserati
cd,
Lorenzo
Maserati
 a,
Tonatiuh
Rangel
ab,
Brett A.
Helms
a,
Tonatiuh
Rangel
ab,
Brett A.
Helms
 ad,
Jeffrey R.
Long
ad,
Jeffrey R.
Long
 cde and
Jeffrey B.
Neaton
*abf
cde and
Jeffrey B.
Neaton
*abf
aMolecular Foundry, Lawrence Berkeley National Laboratory, Berkeley, California 94720, USA. E-mail: jbneaton@lbl.gov
bDepartment of Physics, University of California, Berkeley, California 94720, USA
cDepartment of Chemistry, University of California, Berkeley, California 94720, USA
dMaterials Sciences Division, Lawrence Berkeley National Laboratory, Berkeley, California 94720, USA
eDepartment of Chemical and Biomolecular Engineering, University of California, Berkeley, California 94720, USA
fKavli Energy Nanosciences Institute at Berkeley, Berkeley, California 94720, USA
First published on 23rd May 2018
Abstract
The family of diamine-appended metal–organic frameworks exemplified by compounds of the type mmen–M2(dobpdc) (mmen = N,N′-dimethylethylenediamine; M = Mg, Mn, Fe, Co, Zn; dobpdc4− = 4,4′-dioxidobiphenyl-3,3′-dicarboxylate) are adsorbents with significant potential for carbon capture, due to their high working capacities and strong selectivity for CO2 that stem from a cooperative adsorption mechanism. Herein, we use first-principles density functional theory (DFT) calculations to quantitatively investigate the role of mmen ligands in dictating the framework properties. Our van der Waals-corrected DFT calculations indicate that electrostatic interactions between ammonium carbamate units significantly enhance the CO2 binding strength relative to the unfunctionalized frameworks. Additionally, our computed energetics show that mmen–M2(dobpdc) materials can selectively adsorb CO2 under humid conditions, in agreement with experimental observations. The calculations further predict an increase of 112% and 124% in the orientationally-averaged Young's modulus E and shear modulus G, respectively, for mmen–Zn2(dobpdc) compared to Zn2(dobpdc), revealing a dramatic enhancement of mechanical properties associated with diamine functionalization. Taken together, our calculations demonstrate how functionalization with mmen ligands can enhance framework gas adsorption and mechanical properties.
1 Introduction
Metal–organic frameworks (MOFs) consist of metal clusters or ions that are joined by organic linkers to form porous network solids with large surface areas, high crystallinity, and, in some cases, redox-active open metal sites.1–10 MOFs are promising for gas storage and separation applications, particularly in the area of carbon capture,11–16 and accordingly have received significant recent attention in the literature. Carbon dioxide is mainly produced from the combustion of fossil fuels, and in 2011 alone such CO2 emissions exceeded 32 Gt.16 It is well-known that CO2 produced by combustion is a major driver of global warming,17,18 contributing to rising sea levels and ocean climate change. Therefore, reducing CO2 emissions is among the most urgent problems facing humanity today.Among numerous MOFs currently under investigation for CO2 capture, frameworks of the type M2(dobdc) (M = Mg, Mn, Fe, Co, Ni, Cu, Zn; dobdc4− = 2,5-dioxidobenzene-1,4-dicarboxylate) have been extensively studied due to their high density of open metal sites, which have been shown to engender high selectivity in the separation of various gas molecules.11,19–28 Previous studies have reported impressive uptake capacities, together with heats of CO2 adsorption at the open metal sites as high as 43.5 kJ mol−1 in Mg2(dobdc).29 Notably, this value can be tuned by metal substitution,28 although the M2(dobdc) family exhibits poor CO2 selectivity in the presence of H2O.30–34 Recently, Mason et al.32 reported equilibrium adsorption isotherms for mixtures of CO2, N2, and H2O, the three most prevalent components of flue gas,35 and showed that the CO2 capture performance of M2(dobdc) (M = Mg, Ni) is significantly diminished under humid conditions, due to preferential binding of H2O over CO2 at the open metal sites. For Ni2(dobdc), CO2 uptake is almost zero in the presence of water.
Although the M2(dobdc) materials do not selectively adsorb CO2 under humid conditions, functionalization of open metal sites in the expanded framework M2(dobpdc) (dobpdc4− = 4,4′-dioxidobiphenyl-3,3′-dicarboxylate) with N,N′-dimethylethylenediamine (mmen) has been shown to enhance both the CO2 affinity and selectivity under humid conditions.36–38 In mmen–M2(dobpdc), diamine molecules are grafted onto the open metal sites and dangle into the pore interiors. Notably, the measured heat of CO2 adsorption in mmen–Mg2(dobpdc) is as high as 71 kJ mol−1, almost 30 kJ mol−1 greater than in Mg(dobdc), and mmen–Mg2(dobpdc) is also stable under humid conditions.32 The impressive CO2 capture performance of mmen–Mg2(dobpdc) and other mmen–M2(dobpdc) frameworks stems from a unique cooperative CO2 capture mechanism, which has been shown to persist even in the presence of H2O, based on infrared spectroscopy measurements.32 Interestingly, multicomponent adsorption measurements additionally show that the amount of adsorbed CO2 from a mixture of CO2, N2, and H2O in mmen–Mg2(dobpdc) is slightly higher than that from pure CO2 in mmen–Mg2(dobpdc).
In view of its exceptional CO2 capture performance, we seek to understand quantitatively the properties of mmen–M2(dobpdc) using accurate first-principle density function theory (DFT) calculations. DFT is the most promising method for studying the adsorption (and related) properties of MOFs at the molecular level, owing to its efficiency and accuracy relative to other quantum mechanical methods. In previous theoretical studies, van der Waals (vdW)-corrected DFT in particular has been shown to accurately predict the binding energies and mechanisms of small gas molecules in MOFs,28,29,31,39–42 including MOFs having localized metal 3d electrons and non-zero spin moments.43,44 Other properties, such as mechanical strength, can be predicted with similar accuracy.45 For mmen–M2(dobpdc), the CO2 adsorption mechanism and CO2 interactions with open metal sites in the form of carbamate have also been successfully studied with DFT methods.37,38,46,47
Prior DFT-based studies notwithstanding,37,38,41,46–51 a complete and detailed understanding of the CO2 adsorption energetics in mmen–M2(dobpdc) is still lacking. In particular, the contributions of vdW dispersion interactions to adsorption enthalpies and related properties of mmen–M2(dobpdc) have yet to quantified, and thus a predictive approach for adsorption energies in these complex systems does not yet exist. Moreover, the strength of electrostatic interactions between the ammonium carbamate units formed upon CO2 adsorption and the effect of such interactions on key macroscopic observables, such as mechanical properties, have yet to be quantified and understood. Here, we use vdW-corrected DFT calculations to demonstrate a quantitative approach to predict binding and formation energies in this important class of MOFs. We further quantify the significant electrostatic interactions between ammonium carbamate units; compute and understand the effect of adsorption on mechanical properties; and determine the binding site of H2O on the ammonium carbamate chains and evaluate the influence of humidity on the overall CO2 performance.
2 Methodology
2.1 Computational details
In order to elucidate the role of mmen ligands in the CO2 capture properties of mmen–M2(dobpdc), we perform first-principles DFT calculations within the generalized gradient approximation (GGA) of Perdew, Burke, and Ernzerhof (PBE).52 We use a plane-wave basis and projector augmented-wave (PAW)53,54 pseudopotentials with the Vienna ab initio Simulation Package (VASP) code.55–58 To include the effect of the vdW dispersive interactions on binding energies and mechanical properties, we perform structural relaxations with vdW dispersion-corrected functionals (vdW-DF2)59 as implemented in VASP. The initial structures for the MOFs we consider here are obtained from previous studies.37,38,51,60 For all calculations, we use (i) a Γ-point sampling of the Brillouin zone and (ii) a 600 eV plane-wave cutoff energy. We explicitly treat two valence electrons for Mg (3s2), seven for Mn (3d54s2), eight for Fe (3d64s2), nine for Co (3d74s2), twelve for Zn (3d104s2), six for O (2s22p4), five for N (2s22p3), four for C (2s22p2), and one for H (1s1). All structural relaxations are performed with a Gaussian smearing of 0.05 eV![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 61 and with the structure constrained to the space group P3221. The computed CO2 binding energies are within 0.4 kJ mol−1 when we relax these symmetry constraints. The ions are relaxed until the Hellmann–Feynman forces are less than 0.02 eV Å−1. The convergence threshold for self-consistency is 10−5 eV. For phonon frequency calculations, we use a more rigorous criterion (10−8 eV) (see Table S4†). Hubbard U values of 5.5 eV, 6.5 eV, and 5.3 eV for Mn, Fe, and Co 3d states are chosen following previous studies for M2(dobdc) (M = Ti, V, Cr, Mn, Fe, Co, Ni, Cu).44 Based on the ground state magnetic structure of Fe2(dobdc),21,62 we use ferromagnetic ordering along the metal oxide chain direction and antiferromagnetic ordering between the chains for mmen–M2(dobpdc) (M = Mn, Fe, Co). Our computed electronic structure and measured adsorption spectra are given in the ESI.†
61 and with the structure constrained to the space group P3221. The computed CO2 binding energies are within 0.4 kJ mol−1 when we relax these symmetry constraints. The ions are relaxed until the Hellmann–Feynman forces are less than 0.02 eV Å−1. The convergence threshold for self-consistency is 10−5 eV. For phonon frequency calculations, we use a more rigorous criterion (10−8 eV) (see Table S4†). Hubbard U values of 5.5 eV, 6.5 eV, and 5.3 eV for Mn, Fe, and Co 3d states are chosen following previous studies for M2(dobdc) (M = Ti, V, Cr, Mn, Fe, Co, Ni, Cu).44 Based on the ground state magnetic structure of Fe2(dobdc),21,62 we use ferromagnetic ordering along the metal oxide chain direction and antiferromagnetic ordering between the chains for mmen–M2(dobpdc) (M = Mn, Fe, Co). Our computed electronic structure and measured adsorption spectra are given in the ESI.†
To compute CO2 binding energies, we optimize mmen–M2(dobpdc) prior to CO2 adsorption (Emmen–MOF), interacting with CO2 in the gas phase (ECO2) within a 15 Å × 15 Å × 15 Å cubic supercell, and mmen–M2(dobpdc) with adsorbed CO2 molecules (ECO2–mmen–MOF) using vdW-corrected DFT. The binding energies (EB) are obtained via the difference
| −EB = ECO2–mmen–MOF − (Emmen–MOF + ECO2). | (1) |
We also consider zero-point energy (ZPE) and thermal energy (TE) corrections to compare computed binding energies with experimentally determined CO2 heats of adsorption, following a previous DFT study.28 We calculate vibrational frequencies of bound CO2, H2O, N2, mmen, and CO2–mmen in the framework; we also compute vibrational frequencies of free CO2, H2O, N2, mmen, and CO2–mmen molecules within a 15 Å × 15 Å × 15 Å cubic supercell. In the former case, we assume that changes in the frequency of framework phonon modes are small relative to those of molecular modes. All ZPE and TE corrections are computed at 298 K. All computed Kohn–Sham energies, vibrational frequencies, ZPE, and TE corrections are given in Tables S2, S3, S5, and S6 in the ESI.†
To calculate mechanical properties, we generate the stress tensor with (i) the Γ-point, (ii) a 1000 eV plane-wave cutoff energy, and (iii) a 0.01 eV Å−1 force criterion. The linear elastic properties are then obtained using Hooke's law, which describes the relationship between stress, σ, and strain, ε:
| σi = Cijεj | (2) |
| Sij = Cij−1. | (3) |
After obtaining Cij and Sij, the orientationally-averaged elastic moduli including Young's modulus E, bulk modulus B, shear modulus G, and Poissons ratio v can be simply estimated using the Voigt–Reuss–Hill (VRH) average.64 The detailed formulae used for all reported quantities in this work are given in the ESI.†
3 Results and discussion
3.1 CO2 binding energies
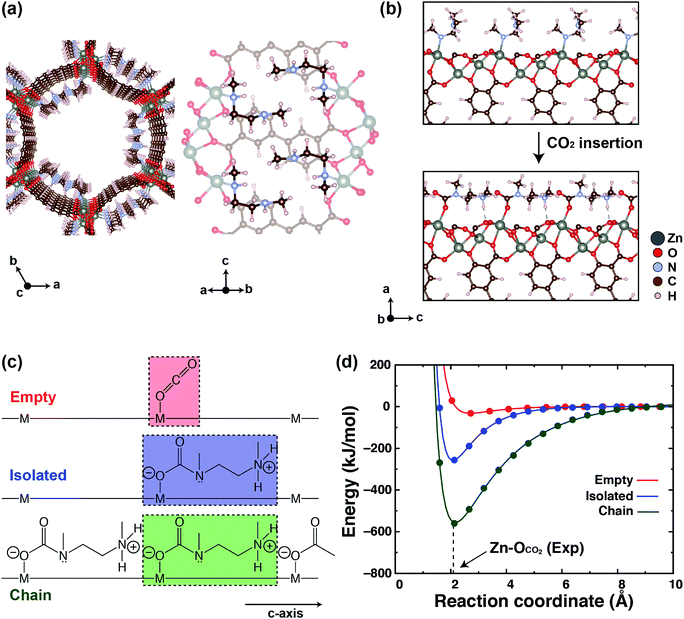
Fig. 1a depicts the optimized crystal structure of mmen–Zn2(dobpdc), which consists of a periodic arrangement of ZnO5 square pyramidal units, dobpdc4− linkers, and mmen ligands crystallizing in the P3221 space group. A right panel of Fig. 1a shows that dangling amines interact with neighbors in the ab-plane via dispersive interactions between methyl groups. These interactions are reflected in the experimentally-determined crystal structure of mmen–Zn2(dobpdc).60 Our calculated lattice parameters for mmen–M2(dobpdc) (M = Mg, Mn, Fe, Co, Zn) are given in Table 1. We do not include results for mmen–Ni2(dobpdc) because the CO2 capture mechanism of this framework is still under investigation. We note that our computed lattice parameters are not in perfect agreement with the experimental values, as expected. As the reparametrized PW86 exchange functional in the vdW-DF2 exchange–correlation functional overestimates repulsive interactions, equilibrium unit cell volumes, and bond distances,59,65,66 the computed lattice parameters and unit cell volumes of M2(dobpdc), CO2–mmen–Mn2(dobpdc), and CO2–mmen–Zn2(dobpdc) are generally larger than the experimental values as shown in Table 1. In addition, our calculations with periodic boundary conditions do not capture any disorder associated with the mmen and CO2–mmen units shown in Fig. S3.† Any degree of disorder in these mmen and CO2–mmen units can alter the average structural properties including the lattice parameters, unit cell volumes, and bond lengths. As example of this, our DFT calculations show that a different mmen ordering of mmen–Fe2(dobpdc) significantly changes the a- and c-axes – by about 0.7 Å and 0.4 Å, respectively – and the unit cell volume by 12.5% (see Fig. S4†). Moreover, according to the previous experimental study by some of us,60 disordered solvent (toluene) is likely present in the pores of the single-crystal structure of mmen–Zn2(dobpdc), for which structural data are collected using a solvated crystal; furthermore, the CO2-inserted single crystal structure of mmen–Zn2(dobpdc) was refined to have 75% occupancy of ammonium carbamate chains, indicating the presence of solvent, CO2, or water at the remaining Zn(II) sites. Hence, these features are mainly responsible for the discrepancies. Nevertheless, despite the presence of some disorder, our vdW-corrected DFT calculations accurately predict the CO2 binding energies (see Table 2) and give the powder diffraction patterns in good overall agreement with the experiments as shown in Fig. S5 and S6.†| M | This work | Experiment | |||||
|---|---|---|---|---|---|---|---|
| Empty | mmen–M | CO2–mmen–M | Empty | mmen–M | CO2–mmen–M | ||
| Mg | a | 22.041 | 21.074 | 21.498 | 21.446 | — | — |
| c | 6.939 | 6.672 | 7.005 | 6.824 | — | — | |
| Mg–N | — | 2.421 | — | — | — | — | |
| Mg–O | — | — | 2.072 | — | — | — | |
| Mn | a | 22.253 | 22.650 | 21.788 | 21.629 | 21.729 | 21.682 |
| c | 7.162 | 6.536 | 7.072 | 6.958 | 7.128 | 7.079 | |
| Mn–N | — | 2.432 | — | — | 2.289 | — | |
| Mn–O | — | — | 2.194 | — | — | 2.097 | |
| Fe | a | 22.230 | 21.387 | 21.890 | 21.848 | — | — |
| c | 6.963 | 6.622 | 7.013 | 6.814 | — | — | |
| Fe–N | — | 2.408 | — | — | — | — | |
| Fe–O | — | — | 2.195 | — | — | — | |
| Co | a | 22.086 | 21.263 | 21.639 | 21.537 | — | — |
| c | 6.920 | 6.540 | 7.005 | 6.798 | — | — | |
| Co–N | — | 2.324 | — | — | — | — | |
| Co–O | — | — | 2.147 | — | — | — | |
| Zn | a | 22.087 | 21.683 | 21.881 | 21.547 | 21.391 | 21.546 |
| c | 6.973 | 7.251 | 6.833 | 6.775 | 6.896 | 6.928 | |
| Zn–N | — | 2.212 | — | — | 2.155 | — | |
| Zn–O | — | — | 2.127 | — | — | 2.087 | |
| This work | Exp | ||||
|---|---|---|---|---|---|
| E B | ZPE | TE | H B | H B | |
| Mg | 74.7 | −2.8 | 1.1 | 73.0 | 71 |
| Mn | 68.9 | −2.2 | 0.6 | 67.3 | 67 |
| Fe | 56.2 | −1.9 | 0.7 | 55.1 | 58 |
| Co | 52.4 | −1.3 | 0.4 | 51.6 | 52 |
| Zn | 62.4 | −1.5 | 1.3 | 62.1 | 57 |
Previous experimental32,37 and computational studies38 demonstrated that mmen–M2(dobpdc) (M = Mg, Mn, Fe, Co, Zn) exhibit step-like isotherms above a threshold CO2 partial pressure, and that these steps originate from a mechanism wherein CO2 gas molecules cooperatively and reversibly insert into metal–mmen bonds followed by formation of a well-ordered ammonium carbamate chain structure, as shown in Fig. 1b. Table 2 compares the computed CO2 binding enthalpies (HB) from this study with those obtained from experiment,37 revealing that our vdW-DF2 calculations can accurately predict CO2 binding enthalpies within ∼5 kJ mol−1. According to our calculations, in all cases MOFs with mmen exhibit an ∼30 kJ mol−1 enhancement in HB compare to the unfunctionalized frameworks (see Tables 2 and 4).
To understand this increase, we performed calculations using three geometries as presented in Fig. 1c. We use the term “Empty” to indicate unappended M2(dobpdc) and “Chain” to indicate the structure with well-ordered ammonium carbamate chains along the channel direction (c-axis in the space group P3221). The term “Isolated” refers to an alternative scenario in which there is no chain formation, and the calculations consider an ammonium carbamate unit bound at just one of the metal sites in the unit cell, with the other metal sites empty.
We then compute the potential interatomic distance curves for the three geometries, varying the separation between CO2 and its binding site, by adopting a a × b × 2c supercell for the isolated and chain geometries. Fig. 1d shows the calculated energy as a function of Zn–OCO2 distance, relative to the experimental Zn–OCO2 distance of 2.087(4) Å for the chain geometry. Similar curves are given for M = Mg, Mn, Fe, Co in Fig. S7 in the ESI.† To calculate these curves, we systematically displace a CO2 molecule and ammonium carbamate along the direction of the ground state Zn–O bond to CO2.
In our DFT calculations, the vdW-DF2 functional slightly overestimates (by ∼2%) the Zn–O distance (2.127 Å) compared with experiment (2.087(4) Å), as reported in previous DFT studies.39,66 In Fig. 1d, the isolated and chain geometries have much deeper binding curves with higher curvature when compared to the empty geometry, reflecting the fact that the binding strength of ammonium carbamate to the MOF interior is much stronger than that of CO2 alone. The binding enthalpy of CO2 in the empty geometry is 29.3 kJ mol−1, which is comparable to the values computed and experimentally measured for Zn2(dobdc) (30.2 kJ mol−1 and 26.8 ± 0.1 kJ mol−1, respectively).28,29 On the other hand, the binding enthalpy of an ammonium carbamate (Hiso) in the isolated geometry is 259.0 kJ mol−1, approximately nine times that of the CO2 binding enthalpy. More interestingly, the ammonium carbamate binding enthalpy (Hchain) in the chain geometry is 562.1 kJ mol−1, which is two times larger than that of the isolated geometry. The other MOFs also show similar behavior (see Fig. S7, ESI†). Thus the magnitude of the electrostatic interaction between two ammonium carbamate units in the chain geometry is equal to 151.5 kJ mol−1 (=(Hchain − Hiso)/2) (because ammonium carbamate unit interacts with two neighboring CO2–mmen sites along the pore axis, we divide by two). This large interaction energy is dominated by the ion pairing and hydrogen bonding interactions of the ammonium group to the unbound oxygen atom of the neighboring carbamate. In magnitude, these interactions are comparable to the heat of formation for ammonium carbamate (∼152 kJ mol−1).67 The results of these calculations show that cooperative CO2 insertion is a very favorable spontaneous process, that the carbamate chain geometry is quite stable, and that electrostatic interactions play a key role in the stability of CO2–mmen–M2(dobpdc) (chain geometry).
The apparent enhancement of CO2 binding strength by the presence of mmen can be qualitatively understood by considering how charges rearrange at the metal sites upon CO2 binding. Fig. 2 shows the charge density difference (Δρ = ρMOF+molecule − (ρMOF + ρmolecule)) isosurface plots for CO2–mmen binding sites in the isolated and chain geometries. The Δρ value calculated for the empty geometry is negligible compared to the values of the isolated and chain geometries at the same isosurface level (see Fig. S8, ESI†). In the isolated geometry, two sites are visible with prominent charge redistribution. One is the Zn–O (carbamate) bond formed upon CO2 insertion into the Zn–mmen bond, and the other is N–H⋯O, which corresponds to hydrogen bonding between the ammonium group of ammonium carbamate and the non-bridging carboxylate oxygen atom on the dobpdc4− linker (Fig. 2a). More interestingly, in the case of the chain geometry there are two additional sites in which ammonium carbamate units interact with their neighbors along the channel direction, as shown in the inset of Fig. 2b. These additional attractive interactions deepen the potential curve compared to the isolated structure (Fig. 1d). Our calculations therefore suggest that strong electrostatic interactions between ammonium carbamate units primarily drive the cooperative CO2 insertion in mmen–M2(dobpdc) and significantly enhance the CO2 binding strength over non-amine-functionalized MOFs.
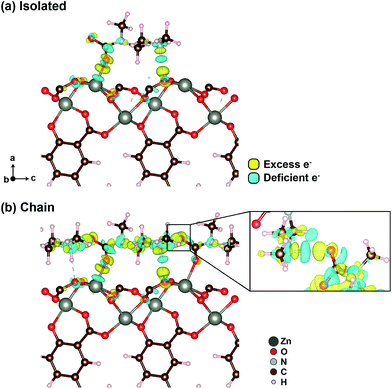 | ||
| Fig. 2 Isosurface plots of the charge density difference (Δρ) for the CO2–mmen binding site in (a) isolated and (b) chain structures. The isosurface level is equal to 0.02 e Å−3. | ||
3.2 Mechanical properties
Having quantified the CO2 binding enhancement afforded by mmen, we now address the mechanical properties of empty and CO2-loaded MOFs, which can be calculated via the elastic modulus C and the curvature of the binding curves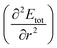 in the linear elastic regime,68,69 where Etot is the total energy and r is reaction coordinate. From the binding energy curves shown in Fig. 1d and S7,† we expect that elastic moduli of mmen–M2(dobpdc) and CO2–mmen–M2(dobpdc) will be much larger than that of M2(dobpdc), and that CO2 adsorption and functionalization can strongly and reversibly alter these quantities. This is because the elastic modulus C is directly proportional to the curvature of the binding curves
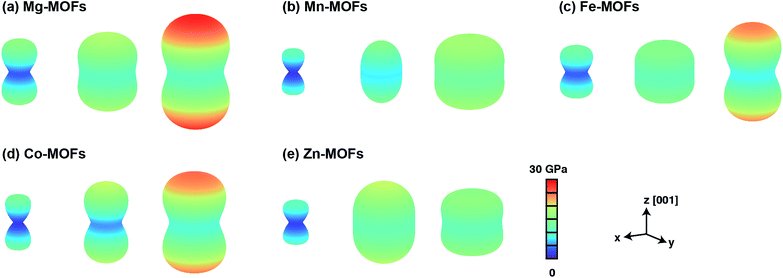
in the linear elastic regime,68,69 where Etot is the total energy and r is reaction coordinate. From the binding energy curves shown in Fig. 1d and S7,† we expect that elastic moduli of mmen–M2(dobpdc) and CO2–mmen–M2(dobpdc) will be much larger than that of M2(dobpdc), and that CO2 adsorption and functionalization can strongly and reversibly alter these quantities. This is because the elastic modulus C is directly proportional to the curvature of the binding curves 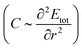 . Our calculations of the Young's modulus E, bulk modulus B, shear modulus G, and Poisson's ratio v for M2(dobpdc), mmen–M2(dobpdc), and CO2–mmen–M2(dobpdc) are summarized in ESI Section 5.† In Fig. 3, we illustrate three-dimensional contours of directionally-dependent values of E for all the frameworks considered. In general, mmen and ammonium carbamate units greatly enhance E along all directions. The most prominent enhancement occurs along the channel direction, along which the ammonium carbamate chains run.
. Our calculations of the Young's modulus E, bulk modulus B, shear modulus G, and Poisson's ratio v for M2(dobpdc), mmen–M2(dobpdc), and CO2–mmen–M2(dobpdc) are summarized in ESI Section 5.† In Fig. 3, we illustrate three-dimensional contours of directionally-dependent values of E for all the frameworks considered. In general, mmen and ammonium carbamate units greatly enhance E along all directions. The most prominent enhancement occurs along the channel direction, along which the ammonium carbamate chains run.
We now examine more quantitatively the effect of mmen and ammonium carbamate units on E, B, G, and v. The orientationally-averaged E, B, G, and v values obtained from eqn (1)–(8) in the ESI† are summarized in Table 3. Averaged over all directions, these values can be considered as elastic moduli of polycrystalline samples with randomly-oriented grains of equal volume fraction. As shown in Fig. 4, E, B, and G are generally larger for mmen–M2(dobpdc) and CO2–mmen–M2(dobpdc) than for M2(dobpdc), an enhancement that can be attributed to mmen and ammonium carbamate units, and this is most pronounced for E and G. For example, the magnitude of E for mmen–Zn2(dobpdc) increases by 112% compared to that of Zn2(dobpdc). More remarkably, the magnitude of E for CO2–mmen–Mn2(dobdpc) increases by 141% compared to that of Mn2(dobpdc) (see Table 3). All E values for mmen–M2(dobdpc) and CO2–mmen–M2(dobpdc) (10.7–16.7 GPa) are higher than the experimental values of conventional frameworks such as MOF-5 (2–8 GPa), HKUST-1 (6 GPa), and ZIFs (2–9 GPa), and lower than that of the hybrid MOF MOFP-1 (23–27 GPa).70–72 For G, the enhancement is even larger than that of E, for instance the G values of mmen–Zn2(dobpdc) and CO2–mmen–Mn2(dobpdc) increase by 124% and 159% compared to Zn2(dobpdc) and Mn2(dobpdc). All G values (4.0–6.4 GPa) are comparable to those of Zr-UiO-67 (5.69 GPa) and Zr-UiO-68 (4.18 GPa).73
| E | B | G | v | Enhancement | ||
|---|---|---|---|---|---|---|
| E | G | |||||
| Mg | 8.97 | 10.05 | 3.32 | 0.35 | ||
| mmen–Mg | 14.10 | 10.49 | 5.53 | 0.28 | 57% | 67% |
| CO2–mmen–Mg | 16.66 | 14.52 | 6.37 | 0.31 | 86% | 92% |
| Mn | 6.42 | 11.98 | 2.28 | 0.41 | ||
| mmen–Mn | 10.72 | 10.36 | 4.04 | 0.33 | 67% | 77% |
| CO2–mmen–Mn | 15.48 | 13.66 | 5.90 | 0.31 | 141% | 159% |
| Fe | 8.63 | 9.69 | 3.19 | 0.35 | ||
| mmen–Fe | 13.56 | 9.55 | 5.37 | 0.26 | 57% | 68% |
| CO2–mmen–Fe | 13.64 | 14.22 | 5.09 | 0.34 | 58% | 59% |
| Co | 6.95 | 8.51 | 2.55 | 0.36 | ||
| mmen–Co | 10.66 | 8.24 | 4.15 | 0.28 | 53% | 63% |
| CO2–mmen–Co | 15.05 | 13.51 | 5.73 | 0.31 | 117% | 125% |
| Zn | 6.89 | 10.28 | 2.48 | 0.39 | ||
| mmen–Zn | 14.58 | 13.03 | 5.55 | 0.31 | 112% | 124% |
| CO2–mmen–Zn | 14.02 | 15.28 | 5.21 | 0.35 | 103% | 110% |
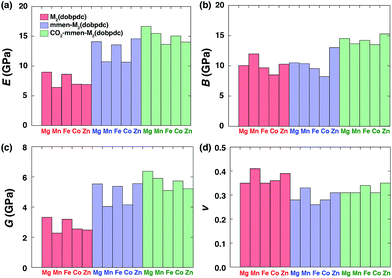 | ||
| Fig. 4 The computed orientationally-averaged (a) Young's modulus E, (b) bulk modulus B, (c) shear modulus G, and (d) Poisson's ratio v values for all frameworks under consideration. | ||
Our calculations thus show that mmen and CO2 binding play a key role in the enhancement of the mechanical properties of the M2(dobpdc) framework materials. Moreover, these results demonstrate that the mechanical properties of MOFs can be tuned by functionalization with ligands such as mmen. Importantly, this bears direct relevance to CO2 capture applications, since carbon capture materials must be mechanically robust and should not exhibit mechanical fatigue during CO2 uptake and release cycling. Relatedly, it has been shown that functionalization with ligands such as mmen can significantly reduce plasticization and enhance selectivity in membrane-type devices.74–76
3.3 CO2 selectivity
In previous multicomponent adsorption measurements incorporating humidity,32 mmen–Mg2(dobpdc) and mmen–Ni2(dobpdc) maintained high CO2 selectivity, while Mg2(dobdc) and Ni2(dobdc) exhibited poor CO2 capture performance under humid conditions. Mason et al. demonstrated that H2O does not alter the cooperative CO2 capture mechanism in mmen–Mg2(dobpdc),32 showing that instead mmen–Mg2(dobpdc) maintains a significant CO2 capacity under multicomponent equilibrium conditions. However, the structural and energetic influence of H2O on CO2 adsorption in mmen–M2(dobpdc) remains unknown.To address this question, we computed HB for CO2, H2O, and N2 in the M2(dobpdc) and mmen–M2(dobpdc) frameworks. Table 4 shows the computed enthalpies of first and second guest molecules (CO2, H2O, and N2) in the frameworks with and without mmen functionalization. In the bare frameworks, water has the highest HB among all three guest molecules at open metal sites. Furthermore, H2O also has the highest HB at secondary binding sites when it has occupied the open metal sites.
| First guest | H B | Second guest | H B | |
|---|---|---|---|---|
| Mg | H2O | 62.6 | H2O | 39.4 |
| CO2 | 25.3 | |||
| N2 | 16.0 | |||
| CO2 | 38.5 | |||
| N2 | 27.3 | |||
| H2O | 40.9 | |||
| CO2–mmen–Mg | 597.0 | CO2 | 26.3 | |
| N2 | 11.2 | |||
| Mn | H2O | 54.6 | H2O | 42.9 |
| CO2 | 26.6 | |||
| N2 | 18.0 | |||
| CO2 | 34.7 | |||
| N2 | 23.6 | |||
| H2O | 43.3 | |||
| CO2–mmen–Mn | 582.2 | CO2 | 29.4 | |
| N2 | 14.6 | |||
| Fe | H2O | 53.9 | H2O | 42.8 |
| CO2 | 20.3 | |||
| N2 | 11.5 | |||
| CO2 | 35.6 | |||
| N2 | 24.6 | |||
| H2O | 40.1 | |||
| CO2–mmen–Fe | 588.4 | CO2 | 25.3 | |
| N2 | 9.5 | |||
| Co | H2O | 53.0 | H2O | 38.7 |
| CO2 | 23.8 | |||
| N2 | 10.9 | |||
| CO2 | 34.9 | |||
| N2 | 22.4 | |||
| H2O | 39.5 | |||
| CO2–mmen–Co | 570.3 | CO2 | 25.7 | |
| N2 | 10.6 | |||
| Zn | H2O | 44.2 | H2O | 38.6 |
| CO2 | 23.2 | |||
| N2 | 16.7 | |||
| CO2 | 29.3 | |||
| N2 | 18.6 | |||
| H2O | 40.5 | |||
| CO2–mmen–Zn | 562.1 | CO2 | 23.7 | |
| N2 | 8.2 |
Fig. 5a shows the most stable computed configuration of Zn2(dobpdc) in the presence of H2O without mmen. Here, the secondary H2O interacts with the first Zn-bound H2O via hydrogen bonding. Previous multicomponent measurements32,77 on the smaller pore MOFs Mg2(dobdc), Co2(dobdc), and Ni2(dobdc) are consistent with this configuration. In ref. 32, in the case of Mg2(dobdc), the amount of CO2 adsorbed was 0.5 mmol g−1, while adsorbed H2O reached over 15 mmol g−1 (1.8 mmol H2O per mmol Mg). Moreover, the amount of CO2 adsorbed in Ni2(dobdc) is almost zero while that of H2O is over 20 mmol g−1 (3.1 mmol H2O per mmol Ni). Therefore, water molecules significantly degrade CO2 selectivity in M2(dobdc) frameworks, and our calculations show that the larger-pore M2(dobpdc) frameworks will exhibit the same behavior (see Table 4).
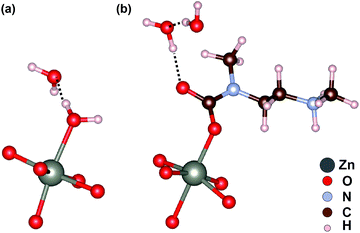 | ||
| Fig. 5 The most stable configurations of (a) Zn2(dobpdc) and (b) mmen–Zn2(dobpdc) in the presence of a mixture of CO2, H2O, and N2. | ||
In contrast, our calculations indicate that CO2 insertion to form an O-bound carbamate for mmen–M2(dobpdc) frameworks is much more favorable than binding H2O at the open metal site of the corresponding bare M2(dobpdc) frameworks. As shown in Table 4, the ammonium carbamate binding enthalpies, Hchain including ZPE and TE corrections, are about ten times higher than those for H2O for all M2(dobpdc) frameworks. Thus, the mmen–M2(dobpdc) frameworks are predicted to maintain their unique CO2 capture mechanism even under humid conditions. Interestingly, our energetics suggest that H2O molecules bind near the negatively charged end of the carbamate via hydrogen bonding interactions (Fig. 5b). This configuration also explains the large amount of H2O adsorbed from the mixture of CO2, H2O, and N2 in CO2–mmen–Mg2(dobpdc).32 In previous multicomponent measurements with mmen–Mg2(dobpdc), the amount of H2O adsorbed from the mixture was about 7 mmol g−1 (1.7 H2O per mmen–Mg), while CO2 adsorbed from the mixture was about 4.2 mmol g−1 (1.0 CO2 per mmen–Mg). In fact, we find that accumulated H2O increases the CO2HB by an amount equal to the H2O binding strength. For example for mmen–Mn2(dobpdc), the binding energy of the second adsorbed H2O molecule hydrogen-bonded to non-bonded O ion on the carbamate via hydrogen bonding (Fig. 5b) is as high as 43.3 kJ mol−1. Thus, the additional hydrogen bonding in the presence of H2O can further stabilize the CO2HB by the same amount (=43.3 kJ mol−1). We speculate that this may be related to an increase of the amount of CO2 adsorbed in the presence of H2O.32 As a result, our computed energetics demonstrate that instead of hampering the CO2 capture process, H2O plays a crucial role in affording stability to CO2–mmen–M2(dobpdc).
4 Conclusions
We have examined the effect of mmen on the binding enthalpies and mechanical properties of mmen–M2(dobpdc) (M = Mg, Mn, Fe, Co, Zn), finding that mmen ligands enhance the CO2HB and selectivity under humid conditions, and that mmen and CO2–mmen ligands significantly enhance framework mechanical properties. These results elucidate the energetics of individual interactions underlying the cooperative CO2 insertion mechanism in mmen–M2(dobpdc) under dry and humid conditions. Furthermore, our results demonstrate the remarkable increase in mechanical properties afforded by functionalization of M2(dobpdc) with diamines. Overall, our work highlights the unique advantages in CO2 capture performance and physical properties accessible with diamine-appended frameworks.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Center for Gas Separations Relevant to Clean Energy Technologies, an Energy Frontier Research Center, funded by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under Award DE-SC0001015. Work at the Molecular Foundry was supported by the Office of Science, Office of Basic Energy Sciences, U.S. Department of Energy, under Contract DE-AC02-05CH11231, and computational resources were provided by DOE (LBNL Lawrencium and NERSC).Notes and references
- A. K. Cheetham, G. Férey and L. Thierry, Angew. Chem., Int. Ed., 1999, 38, 3268–3292 CrossRef PubMed.
- S. L. James, Chem. Soc. Rev., 2003, 32, 276–288 RSC.
- H. C. Zhou, J. R. Long and O. M. Yaghi, Chem. Rev., 2012, 112, 673–674 CrossRef PubMed.
- J. L. C. Rowsell and O. M. Yaghi, Microporous Mesoporous Mater., 2004, 73, 3–14 CrossRef.
- H. Furukawa, K. Cordova, M. O'Keeffe and O. M. Yaghi, Science, 2013, 341, 1230444 CrossRef PubMed.
- M. L. Foo, R. Matsuda and S. Kitagawa, Chem. Mater., 2014, 26, 310–322 CrossRef.
- G. Férey, Dalton Trans., 2009, 4400–4415 RSC.
- O. K. Farha and J. T. Hupp, Acc. Chem. Res., 2010, 43, 1166–1175 CrossRef PubMed.
- K. K. Tanabe and S. M. Cohen, Chem. Soc. Rev., 2011, 40, 498–519 RSC.
- J. R. Long and O. M. Yaghi, Chem. Soc. Rev., 2009, 38, 1213–1214 RSC.
- N. L. Rosi, J. Eckert, M. Eddaoudi, D. T. Vodak, J. Kim, M. O'Keeffe and O. M. Yaghi, Science, 2003, 73, 12–15 Search PubMed.
- A. R. Millward and O. M. Yaghi, J. Am. Chem. Soc., 2005, 127, 17998–17999 CrossRef PubMed.
- K. Sumida, D. L. Rogow, J. A. Mason, T. M. McDonald, E. D. Bloch, Z. R. Herm, T.-H. Bae and J. R. Long, Chem. Rev., 2012, 112, 724–781 CrossRef PubMed.
- J. Liu, P. K. Thallapally, B. P. McGrail, D. R. Brown and J. Liu, Chem. Soc. Rev., 2012, 41, 2308–2322 RSC.
- T. A. Makal, J.-R. Li, W. Lu and H.-C. Zhou, Chem. Soc. Rev., 2012, 41, 7761–7779 RSC.
- International Energy Agency, CO2 Emissions From Fuel Combustion Highlights, 2015 Search PubMed.
- F. Joos, G.-K. Plattner, T. F. Stocker, O. Marchal and A. Schmittner, Science, 1999, 284, 464–467 CrossRef PubMed.
- P. M. Cox, R. A. Betts, C. D. Jones, S. A. Spall and J. Totterdell, Nature, 2000, 408, 184–187 CrossRef PubMed.
- W. Zhou, H. Wu and T. Yildirim, J. Am. Chem. Soc., 2008, 2, 15268–15269 CrossRef PubMed.
- S. R. Caskey, A. G. Wong-Foy and A. J. Matzger, J. Am. Chem. Soc., 2008, 130, 10870–10871 CrossRef PubMed.
- E. D. Bloch, L. J. Murray, W. L. Queen, S. Chavan, S. N. Maximoff, J. P. Bigi, R. Krishna, V. K. Peterson, F. Grandjean, G. J. Long, B. Smit, S. Bordiga, C. M. Brown and J. R. Long, J. Am. Chem. Soc., 2011, 133, 14814–14822 CrossRef PubMed.
- R. Sanz, F. Martínez, G. Orcajo, L. Wojtas and D. Briones, Dalton Trans., 2013, 42, 2392–2398 RSC.
- W. L. Queen, C. M. Brown, D. K. Britt, P. Zajdel, M. R. Hudson and O. M. Yaghi, J. Phys. Chem. C, 2011, 115, 24915–24919 Search PubMed.
- D. J. Xiao, E. D. Bloch, J. A. Mason, W. L. Queen, M. R. Hudson, N. Planas, J. Borycz, A. L. Dzubak, P. Verma, K. Lee, F. Bonino, V. Crocella, J. Yano, S. Bordiga, D. G. Truhlar, L. Gagliardi, C. M. Brown and J. R. Long, Nat. Chem., 2014, 6, 590–595 CrossRef PubMed.
- P. D. C. Dietzel, Y. Morita, R. Blom and H. Fjellvåg, Angew. Chem., Int. Ed., 2005, 44, 6354–6358 CrossRef PubMed.
- P. D. C. Dietzel, B. Panella, M. Hirscher, R. Blom and H. Fjellvåg, Chem. Commun., 2006, 1, 959–961 RSC.
- Y. Liu, H. Kabbour, C. M. Brown, D. A. Neumann and C. C. Ahn, Langmuir, 2008, 24, 4772–4777 CrossRef PubMed.
- K. Lee, J. D. Howe, L.-C. Lin, B. Smit and J. B. Neaton, Chem. Mater., 2015, 27, 668–678 CrossRef.
- W. L. Queen, M. R. Hudson, E. D. Bloch, J. A. Mason, M. I. Gonzalez, J. S. Lee, D. Gygi, J. D. Howe, K. Lee, T. A. Darwish, M. James, V. K. Peterson, S. J. Teat, B. Smit, J. B. Neaton, J. R. Long and C. M. Brown, Chem. Sci., 2014, 5, 4569–4581 RSC.
- J. Yu and P. B. Balbuena, J. Phys. Chem. C, 2013, 117, 3383–3388 Search PubMed.
- K. Tan, S. Zuluaga, Q. Gong, Y. Gao, N. Nijem, J. Li, T. Thonhauser and Y. J. Chabal, Chem. Mater., 2015, 27, 2203–2217 CrossRef.
- J. A. Mason, T. M. McDonald, T.-H. Bae, J. E. Bachman, K. Sumida, J. J. Dutton, S. S. Kaye and J. R. Long, J. Am. Chem. Soc., 2015, 137, 4787–4803 CrossRef PubMed.
- S. Zuluaga, E. M. A. Fuentes-Fernandez, K. Tan, F. Xu, J. Li, Y. J. Chabal and T. Thonhauser, J. Mater. Chem. A, 2016, 4, 5176–5183 Search PubMed.
- J. Kundu, T. Pascal, D. Prendergast and S. Whitelam, Phys. Chem. Chem. Phys., 2016, 18, 21760–21766 RSC.
- T. C. Drage, C. E. Snape, L. A. Stevens, J. Wood, J. Wang, A. I. Cooper, R. Dawson, X. Guo, C. Satterley and R. Irons, J. Mater. Chem., 2012, 22, 2815–2823 RSC.
- T. M. McDonald, W. R. Lee, J. a. Mason, B. M. Wiers, C. S. Hong and J. R. Long, J. Am. Chem. Soc., 2012, 134, 7056–7065 CrossRef PubMed.
- T. M. McDonald, J. A. Mason, X. Kong, E. D. Bloch, D. Gygi, A. Dani, V. Crocellà, F. Giordanino, S. O. Odoh, W. S. Drisdell, B. Vlaisavljevich, A. L. Dzubak, R. Poloni, S. K. Schnell, N. Planas, K. Lee, T. Pascal, L. F. Wan, D. Prendergast, J. B. Neaton, B. Smit, J. B. Kortright, L. Gagliardi, S. Bordiga, J. A. Reimer and J. R. Long, Nature, 2015, 519, 303–308 CrossRef PubMed.
- B. Vlaisavljevich, S. O. Odoh, S. K. Schnell, A. L. Dzubak, K. Lee, N. Planas, J. B. Neaton, L. Gagliardi and B. Smit, Chem. Sci., 2015, 6, 5177–5185 RSC.
- R. Poloni, B. Smit and J. B. Neaton, J. Phys. Chem. A, 2012, 116, 4957–4964 CrossRef PubMed.
- P. Canepa, N. Nijem, Y. J. Chabal and T. Thonhauser, Phys. Rev. Lett., 2013, 110, 026102 CrossRef PubMed.
- W. S. Drisdell, R. Poloni, T. M. McDonald, J. R. Long, B. Smit, J. B. Neaton, D. Prendergast and J. B. Kortright, J. Am. Chem. Soc., 2013, 135, 18183–18190 CrossRef PubMed.
- B. Vlaisavljevich, J. Huck, Z. Hulvey, K. Lee, J. A. Mason, J. B. Neaton, J. R. Long, C. M. Brown, D. Alfé, A. Michaelides and B. Smit, J. Phys. Chem. A, 2017, 121, 4139–4151 CrossRef PubMed.
- T. Thonhauser, S. Zuluaga, C. A. Arter, K. Berland, E. Schröder and P. Hyldgaard, Phys. Rev. Lett., 2015, 115, 136402 CrossRef PubMed.
- G. W. Mann, K. Lee, M. Cococcioni, B. Smit and J. B. Neaton, J. Chem. Phys., 2016, 144, 174104 CrossRef PubMed.
- J.-C. Tan, B. Civalleri, C.-C. Lin, L. Valenzano, R. Galvelis, P.-F. Chen, T. D. Bennett, C. Mellot-Draznieks, C. M. Zicovich-Wilson and A. K. Cheetham, Phys. Rev. Lett., 2012, 108, 095502 CrossRef PubMed.
- N. Planas, A. L. Dzubak, R. Poloni, L. C. Lin, A. McManus, T. M. McDonald, J. B. Neaton, J. R. Long, B. Smit and L. Gagliardi, J. Am. Chem. Soc., 2013, 135, 7402–7405 CrossRef PubMed.
- W. S. Drisdell, R. Poloni, T. M. McDonald, T. A. Pascal, L. F. Wan, C. D. Pemmaraju, B. Vlaisavljevich, S. O. Odoh, J. B. Neaton, J. R. Long, D. Prendergast and J. B. Kortright, Phys. Chem. Chem. Phys., 2015, 17, 21448–21457 RSC.
- S. A. Didas, M. A. Sakwa-novak, G. S. Foo, C. Sievers and C. W. Jones, J. Phys. Chem. Lett., 2014, 5, 4194–4200 CrossRef PubMed.
- W. R. Lee, H. Jo, L.-M. Yang, H. Lee, D. W. Ryu, K. S. Lim, J. H. Song, D. Y. Min, S. S. Han, J. G. Seo, Y. K. Park, D. Moon and C. S. Hong, Chem. Sci., 2015, 6, 3697–3705 RSC.
- L. A. Darunte, K. S. Walton, D. S. Sholl and C. W. Jones, Curr. Opin. Chem. Eng., 2016, 12, 82–90 CrossRef.
- D. Gygi, E. D. Bloch, J. A. Mason, M. R. Hudson, M. I. Gonzalez, R. L. Siegelman, T. A. Darwish, W. L. Queen, C. M. Brown and J. R. Long, Chem. Mater., 2016, 28, 1128–1138 CrossRef.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef PubMed.
- P. E. Blöchl, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 50, 17953–17979 CrossRef.
- G. Kresse and D. Joubert, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 59, 1758–1775 CrossRef.
- G. Kresse and J. Hafner, Phys. Rev. B: Condens. Matter Mater. Phys., 1993, 47, 558–561 CrossRef.
- G. Kresse and J. Furthmüller, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54, 11169–11186 CrossRef.
- G. Kresse and J. Furthmüller, Comput. Mater. Sci., 1996, 6, 15–50 CrossRef.
- G. Kresse and J. Hafner, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 49, 14251–14269 CrossRef.
- K. Lee, É. D. Murray, L. Kong, B. I. Lundqvist and D. C. Langreth, Phys. Rev. B: Condens. Matter Mater. Phys., 2010, 82, 081101 CrossRef.
- R. L. Siegelman, T. M. McDonald, M. I. Gonzalez, J. D. Martell, P. J. Milner, J. A. Mason, A. H. Berger, A. S. Bhown and J. R. Long, J. Am. Chem. Soc., 2017, 139, 10526–10538 CrossRef PubMed.
- C. Elsässer, M. Fähnle, C. Chan and K. Ho, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 49, 13975–13978 CrossRef.
- E. D. Bloch, W. L. Queen, R. Krishna, J. M. Zubairy, C. M. Brown and J. R. Long, Science, 2012, 335, 1606–1611 CrossRef PubMed.
- Y. Le Page and P. Saxe, Phys. Rev. B: Condens. Matter Mater. Phys., 2002, 65, 104104 CrossRef.
- R. Hill, Proc. Phys. Soc., London, Sect. A, 1952, 65, 349–354 CrossRef.
- É. D. Murray, K. Lee and D. C. Langreth, J. Chem. Theory Comput., 2009, 5, 2754–2762 CrossRef PubMed.
- I. Hamada, Phys. Rev. B: Condens. Matter Mater. Phys., 2014, 89, 121103 CrossRef.
- K. G. Clark and H. C. Hetherington, J. Am. Chem. Soc., 1927, 49, 1909–1915 CrossRef.
- D. A. Padmavathi, Mater. Sci. Appl., 2011, 22, 97–104 Search PubMed.
- Handbook of Advanced Ceramics, ed. S. Somiya, Academic Press, 2013 Search PubMed.
- J. C. Tan, T. D. Bennett and A. K. Cheetham, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 9938–9943 CrossRef PubMed.
- J. C. Tan and A. K. Cheetham, Chem. Soc. Rev., 2011, 40, 1059–1080 RSC.
- W. Li, A. Thirumurugan, P. T. Barton, Z. Lin, S. Henke, H. H. M. Yeung, M. T. Wharmby, E. G. Bithell, C. J. Howard and A. K. Cheetham, Phys. Rev. B: Condens. Matter Mater. Phys., 2014, 136, 7801–7804 Search PubMed.
- H. Wu, T. Yildirim and W. Zhou, J. Phys. Chem. Lett., 2013, 4, 925–930 CrossRef PubMed.
- J. E. Bachman, Z. P. Smith, T. Li, T. Xu and J. R. Long, Nat. Mater., 2016, 15, 845–851 CrossRef PubMed.
- J. E. Bachman and J. R. Long, Energy Environ. Sci., 2016, 9, 2031–2036 Search PubMed.
- L. Maserati, S. M. Meckler, J. E. Bachman, J. R. Long and B. A. Helms, Nano Lett., 2017, 17, 6828–6832 CrossRef PubMed.
- R. Mercado, B. Vlaisavljevich, L.-C. Lin, K. Lee, Y. Lee, J. A. Mason, D. J. Xiao, M. I. Gonzalez, M. T. Kapelewski, J. B. Neaton and B. Smit, J. Phys. Chem. C, 2016, 120, 12590–12604 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7sc05217k |
| This journal is © The Royal Society of Chemistry 2018 |