Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Exploiting the interactions between the ruthenium Hoveyda–Grubbs catalyst and Al-modified mesoporous silica: the case of SBA15 vs. KCC-1†
Baraa
Werghi‡
a,
Eva
Pump‡
 a,
Mykyta
Tretiakov
a,
Edy
Abou-Hamad
b,
Andrei
Gurinov
a,
Mykyta
Tretiakov
a,
Edy
Abou-Hamad
b,
Andrei
Gurinov
 b,
Pradeep
Doggali
b,
Pradeep
Doggali
 a,
Dalaver H.
Anjum
b,
Luigi
Cavallo
a,
Dalaver H.
Anjum
b,
Luigi
Cavallo
 a,
Anissa
Bendjeriou-Sedjerari
a,
Anissa
Bendjeriou-Sedjerari
 *a and
Jean-Marie
Basset
*a and
Jean-Marie
Basset
 *a
*a
aKAUST Catalysis Center (KCC), King Abdullah University of Science and Technology, Thuwal, 23955-6900, Saudi Arabia. E-mail: jeanmarie.basset@kaust.edu.sa; Anissa.BendjeriouSedjerari@kaust.edu.sa
bKing Abdullah University of Science and Technology (KAUST), Core Labs, Thuwal, 23955-6900, Saudi Arabia
First published on 5th March 2018
Abstract
Immobilization of the 2nd generation Hoveyda–Grubbs catalyst HG-II onto well-ordered 2D hexagonal (SBA15) and 3D fibrous (KCC-1) mesostructured silica, which contained tetra-coordinated Al, has been investigated through the Surface Organometallic Chemistry (SOMC) methodology. The main interest of this study lies in the peculiarity of the silica supports, which display a well-defined tetrahedral aluminum hydride site displaying a strong Lewis acid character, [(![[triple bond, length half m-dash]](https://www.rsc.org/images/entities/char_e007.gif) Si–O–Si
Si–O–Si![[triple bond, length half m-dash]](https://www.rsc.org/images/entities/char_e007.gif) )(
)(![[triple bond, length half m-dash]](https://www.rsc.org/images/entities/char_e007.gif) Si–O–)2Al–H]. The resulting supported Hoveyda–Grubbs catalysts have been fully characterized by advanced solid state characterization techniques (FT-IR, 1H and 13C solid state NMR, DNP-SENS, EF-TEM…). Together with DFT calculations, the immobilization of HG-II does not occur through the formation of a covalent bond between the complex and the Al-modified mesoporous silica as expected, but through an Al⋯Cl–[Ru]-coordination. It is not surprising that in functionalized olefin metathesis of diethyldiallyl malonate, DEDAM (liquid phase), leaching of the catalyst is observed which is not the case in non-functionalized olefin metathesis of propene (gas phase). Besides, the results obtained in propene metathesis with HG-II immobilized either on SBA15 (dpore = 6 nm) or KCC-1 (dpore = 4 or 8 nm) highlight the importance of the accessibility of the catalytic site. Therefore, we demonstrate that KCC-1 is a promising and suitable 3D mesoporous support to overcome the diffusion of reactants into the porous network of heterogeneous catalysts.
Si–O–)2Al–H]. The resulting supported Hoveyda–Grubbs catalysts have been fully characterized by advanced solid state characterization techniques (FT-IR, 1H and 13C solid state NMR, DNP-SENS, EF-TEM…). Together with DFT calculations, the immobilization of HG-II does not occur through the formation of a covalent bond between the complex and the Al-modified mesoporous silica as expected, but through an Al⋯Cl–[Ru]-coordination. It is not surprising that in functionalized olefin metathesis of diethyldiallyl malonate, DEDAM (liquid phase), leaching of the catalyst is observed which is not the case in non-functionalized olefin metathesis of propene (gas phase). Besides, the results obtained in propene metathesis with HG-II immobilized either on SBA15 (dpore = 6 nm) or KCC-1 (dpore = 4 or 8 nm) highlight the importance of the accessibility of the catalytic site. Therefore, we demonstrate that KCC-1 is a promising and suitable 3D mesoporous support to overcome the diffusion of reactants into the porous network of heterogeneous catalysts.
1 Introduction
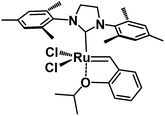
Regarding the development of homogeneous olefin metathesis, ruthenium(II) catalysts have impacted on numerous applications ranging from industrial processes involving polymers, pharmaceuticals and fine chemicals.1–5 One of the most eminent homogeneous catalysts to perform olefin metathesis reactions is the second generation Hoveyda–Grubbs catalyst, HG-II,6 bearing a Ru(II) metal centre surrounded by an N-heterocyclic carbene (NHC), two anionic chlorine ligands and one chelating benzylidene ligand containing an ether functionality coordinated to Ru (Fig. 1).1,7,8This catalytic system is particularly efficient for metathesis reactions involving highly electron-deficient substrates. The most significant breakthrough of the HG-II catalyst is its tolerance to functional groups and therefore its ability to perform the metathesis of functional olefins,9 ring closing metathesis (RCM), ring opening metathesis polymerization (ROMP) and cross metathesis (CM).3,10–15 Despite its impressive catalytic activity, versatility and stability, shortcomings still need to be resolved including easy separation from the reaction medium, recyclability and bimolecular decomposition of the homogeneous catalyst.16,17 These issues can be overcome by immobilization of HG-II on solid supports. To date, many attempts using the supported homogeneous catalysis strategy over hybrid mesoporous silica have been made,18–21 but this approach leads to ill-defined supported homogeneous catalysts which are different from heterogeneous catalysts as the complexes may interact further with the surface.22–25 Recently, some groups demonstrated that a simple adsorption of HG-II onto different mesoporous silicas (SBA15, MCM41, SBA1…) induces a confinement of the Ru complex (size of HG-II: 1.76 × 1.37 × 1.047 nm3)26 inside the mesopores (from 1.5 to 6 nm) and therefore enables recyclability of the solid catalyst for olefin metathesis.26–28 In this case, the polarity of the solvent is crucial; lower polarity is preferred to avoid leaching of the Ru complex from the solid. However, the type of interaction between the Ru catalyst and the silica support remains unresolved, preventing the establishment of a clear anchoring mechanism of the complex onto the support. As a consequence, one would wonder whether the silica supported Ru retains its own activity and if the catalyst acts as a true heterogeneous catalyst.
The Surface Organometallic Chemistry (SOMC) methodology is a well-established approach to design well-defined single site supported catalysts featuring truly heterogeneous activity.29 The strategy is based on the reaction of a given organometallic compound (e.g. WMe6, TaMe5…) with isolated silanol of highly dehydroxylated silica and leads to the formation of a surface organometallic fragment (SOMF).29,30 However, the design of well-defined and single site ruthenium based SOMFs is difficult mainly due to the low affinity of Ru(II) complexes towards oxygen containing ligands. Recently, we succeeded in developing a novel type of chemically modified support for SOMC applications.31–33 Among them, a well-defined tetrahedral aluminum hydride site, [(![[triple bond, length half m-dash]](https://www.rsc.org/images/entities/char_e007.gif) Si–O–Si
Si–O–Si![[triple bond, length half m-dash]](https://www.rsc.org/images/entities/char_e007.gif) )(
)(![[triple bond, length half m-dash]](https://www.rsc.org/images/entities/char_e007.gif) Si–O–)2Al–H], [Al–H], was synthesized by reaction of di-isobutyl aluminum hydride (DIBAL) with the isolated silanol of a dehydroxylated SBA15 (700 °C, 10−5 mbar).34
Si–O–)2Al–H], [Al–H], was synthesized by reaction of di-isobutyl aluminum hydride (DIBAL) with the isolated silanol of a dehydroxylated SBA15 (700 °C, 10−5 mbar).34
In this work, we were interested to study the type of interaction between HG-II and the supported Lewis acid [Al–H] site grafted onto two types of modified mesoporous silica, SBA15 and KCC-1. Combining advanced solid state spectroscopies (FT-IR, SS NMR, DNP-SENS, EF-TEM…) and DFT calculations, we were able to propose an anchoring mechanism between HG-II and the Al-modified mesoporous silica. We will show that an Al⋯Cl–[Ru] interaction is responsible for the immobilization of HG-II. In parallel, we will demonstrate how the mesostructure of the silica (2D vs. 3D and pore diameter) affects the catalytic activity in propene metathesis.
2 Results and discussion
Generation of [Al–H] surface groups on well-ordered mesoporous silica SBA15 and KCC-1
Two types of mesoporous materials were chosen: SBA15 (dpore = 6 nm)35 and KCC-1 (dpore = 8 and 4 nm).36,37 SBA15 is one of the most used mesoporous silicas in the field of heterogeneous catalysis. It was chosen because of its following structural parameters: 2D well-ordered hexagonal mesostructure, high surface area (800 m2 g−1), large uniform pore diameter (6 nm), and thermal (up to 1200 °C) and mechanical stability.35 However, it has been demonstrated that the accessibility of the active site and/or a hindered diffusion (of the substrate and products) inside the mesopores remains an important issue which needs to be resolved. To overcome these limitations, 3D well-ordered mesoporous materials appear to be the ideal candidates as these supports avoid diffusion issues and provide better active site accessibility. In 2010, our group developed a new family of high-surface area silica nano-spheres, KCC-1, with a spectacular 3D fibrous morphology combined with high surface area (>600 m2 g−1), high range of particle size (170–1120 nm),37 and high thermal, chemical and mechanical stability. The structural parameters, surface area and pore size of both parent materials, SBA15 and KCC-1, are given in the ESI (Fig. S7–S9, ESI).† All materials exhibit a well-ordered mesoporous structure according to their nitrogen sorption isotherms. Transmission Electron Microscopy (TEM) clearly shows a 2D hexagonal structure for SBA15 (Fig. 6a) while KCC-1 is characterized by a 3D fibrous morphology (Fig. 6f). Hence, the morphology of the materials, their structure and hierarchical organization (2D or 3D networks, shape…) might affect the catalytic activity based on the accessibility of the active sites.The generation of well-defined tetrahedral aluminum hydride [Al–H] on SBA15700 (dpore = 6 nm), A0, KCC-1700 (dpore = 8 nm), B0, and KCC-1700 (dpore = 4 nm), C0, was achieved as previously described in the literature.34,36,37 It consists of a dehydroxylation pretreatment of mesoporous silica (700 °C, 10−5 mbar, 30 h), which leads to the formation of isolated silanol (![[triple bond, length half m-dash]](https://www.rsc.org/images/entities/char_e007.gif) SiOH) (ESI†).
SiOH) (ESI†).
The reaction of dehydroxylated mesoporous silica A0, B0 and C0 with DIBAL (1 eq. per [![[triple bond, length half m-dash]](https://www.rsc.org/images/entities/char_e007.gif) SiOH]) leads to a bipodal well-defined single-site tetrahedral iso-butylaluminum supported complex, [(
SiOH]) leads to a bipodal well-defined single-site tetrahedral iso-butylaluminum supported complex, [(![[triple bond, length half m-dash]](https://www.rsc.org/images/entities/char_e007.gif) Si–O–Si
Si–O–Si![[triple bond, length half m-dash]](https://www.rsc.org/images/entities/char_e007.gif) )(
)(![[triple bond, length half m-dash]](https://www.rsc.org/images/entities/char_e007.gif) Si–O–)2Al–iBu], 1a, along with silicon hydride, [
Si–O–)2Al–iBu], 1a, along with silicon hydride, [![[triple bond, length half m-dash]](https://www.rsc.org/images/entities/char_e007.gif) Si–H], 1b, and silicon isobutyl, [
Si–H], 1b, and silicon isobutyl, [![[triple bond, length half m-dash]](https://www.rsc.org/images/entities/char_e007.gif) Si–CH2CH(CH3)2], 1c.
Si–CH2CH(CH3)2], 1c.
[Al–H], 2a, the hydride homologue of 1a, is obtained by a simple thermal treatment (ESI†) obtained through a β-H elimination from the –CH3 of the isobutyl moiety to the Al-center (Scheme 1). Advanced solid state characterization (1H, 13C, 27Al and 29Si SS NMR and FT-IR) and DFT calculations provided clear knowledge of the atomic composition of the surface site, which is essential to establishing structure–activity relationships at the molecular and atomic levels.33,34
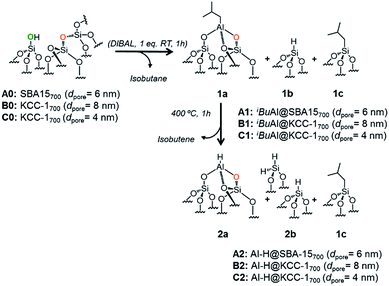 | ||
| Scheme 1 Reaction of DIBAL with A0, B0 and C0 (1 eq. DIBAL/silanol) to yield A1, B1 and C1 followed by thermal treatment leading to A2, B2 and C2. | ||
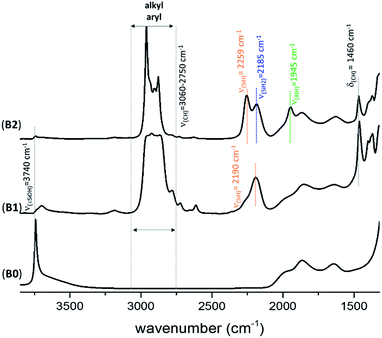
The FT-IR spectra of KCC-1 B2 (Fig. 2) and C2 (Fig. S4, ESI†) are similar to the one previously obtained with SBA15 A2 (Fig. S1, ESI†). Fig. 2 shows the FT-IR spectrum of B0, B1 and B2 after each step of synthesis (dehydroxylation at 700 °C for 30 h, reaction with DIBAL for 1 h and thermal treatment at 400 °C for 1 h, respectively). The characteristic vibrational band of isolated silanol, ν(OH) at 3747 cm−1, instantaneously disappears after reaction with DIBAL (B1, Fig. 2). Meanwhile, the alkyl vibrational bands ν(CH) of [(![[triple bond, length half m-dash]](https://www.rsc.org/images/entities/char_e007.gif) Si–O–Si
Si–O–Si![[triple bond, length half m-dash]](https://www.rsc.org/images/entities/char_e007.gif) )(
)(![[triple bond, length half m-dash]](https://www.rsc.org/images/entities/char_e007.gif) Si–O–)2Al–iBu] and [
Si–O–)2Al–iBu] and [![[triple bond, length half m-dash]](https://www.rsc.org/images/entities/char_e007.gif) Si–iBu] appear from 3060 to 2750 cm−1. The FT-IR band at 2190 cm−1ν(Si–H) is assigned to silicon monohydride.31,33,34
Si–iBu] appear from 3060 to 2750 cm−1. The FT-IR band at 2190 cm−1ν(Si–H) is assigned to silicon monohydride.31,33,34
The generation of the terminal [(![[triple bond, length half m-dash]](https://www.rsc.org/images/entities/char_e007.gif) Si–O–Si
Si–O–Si![[triple bond, length half m-dash]](https://www.rsc.org/images/entities/char_e007.gif) )(
)(![[triple bond, length half m-dash]](https://www.rsc.org/images/entities/char_e007.gif) Si–O–)2Al–H] is characterized by the presence of the band ν(Al–H) at 1945 cm−1. The vibrational bands at 2259 and 2185 cm−1 are characteristic of ν(SiH) and ν(SiH2), respectively.33,34 Moreover, the alkyl vibrational bands of (
Si–O–)2Al–H] is characterized by the presence of the band ν(Al–H) at 1945 cm−1. The vibrational bands at 2259 and 2185 cm−1 are characteristic of ν(SiH) and ν(SiH2), respectively.33,34 Moreover, the alkyl vibrational bands of (![[triple bond, length half m-dash]](https://www.rsc.org/images/entities/char_e007.gif) Si–iBu) in the region between ν(CH) = 3024 and 2823 cm−1 and the corresponding stretching bands ν(CH) = 1460 cm−1 and 1380 cm−1 maintain their respective frequency and intensity, showing that these alkyl groups remain on the surface.
Si–iBu) in the region between ν(CH) = 3024 and 2823 cm−1 and the corresponding stretching bands ν(CH) = 1460 cm−1 and 1380 cm−1 maintain their respective frequency and intensity, showing that these alkyl groups remain on the surface.
Interestingly, the [Al–H] surface groups have been identified as strong Lewis acid sites through the adsorption/desorption of pyridine, pKb = 5.21 (ESI†). Indeed, upon exposure to pyridine followed by evacuation at 400 °C at 10−5 mbar, the FT-IR spectrum displays three vibrational bands at 1455, 1578 and 1622 cm−1. These bands are assigned to the interaction between the lone pair of pyridine and the vacant orbital of Lewis acid sites, [Al–H] (Fig. S2, ESI†). We demonstrated by DFT calculations that the coordination of pyridine is favoured by −12.8 kcal mol−1. The aluminium orbital is accessible for the doublet of the nitrogen atom of pyridine as the coordination bond between the siloxane bridge [(![[triple bond, length half m-dash]](https://www.rsc.org/images/entities/char_e007.gif) Si–O–Si
Si–O–Si![[triple bond, length half m-dash]](https://www.rsc.org/images/entities/char_e007.gif) )] and the Al–H group from [(
)] and the Al–H group from [(![[triple bond, length half m-dash]](https://www.rsc.org/images/entities/char_e007.gif) Si–O–Si
Si–O–Si![[triple bond, length half m-dash]](https://www.rsc.org/images/entities/char_e007.gif) )(
)(![[triple bond, length half m-dash]](https://www.rsc.org/images/entities/char_e007.gif) Si–O)2 Al–H] is released, which is shown by an increased Al⋯O distance from 2.02 Å to 3.22 Å.
Si–O)2 Al–H] is released, which is shown by an increased Al⋯O distance from 2.02 Å to 3.22 Å.
Immobilization of the second generation Hoveyda–Grubbs ruthenium catalyst onto [Al–H] modified SBA15 and KCC-1
Reactions of complex HG-II with A2, B2 and C2 were conducted at room temperature in dichloromethane (DCM) for 5 h (ESI†). The amount introduced was 0.2 eq. [Ru]/[Al–H]. Above this value, the grafting is not complete. During the reaction, we clearly observe a colour change of the solution (from green to colourless) and the resulting materials A3, B3 and C3 become brown. Previously, it was mentioned that a reaction of HG-II with alumina might result in an immediate decomposition of the complex induced through Lewis acidic sites.28 This hypothesis could be disproved through the following study of A3, B3 and C3 by FT-IR, SS NMR, and DNP SENS characterization and catalytic tests. During the reaction of SBA15700, A0 (blank experiment), with HG-II, the solution remains green and the materials turn light green.The FT-IR spectra of A3, B3 and C3 (Fig. S3, 3 and S4,† respectively) show the appearance of new alkyl and aryl bands in the region between 3050 and 2800 cm−1 and 1608 cm−1, and their stretching bands δ(CH) at 1461 cm−1 and 1380 cm−1. The appearance of a small shoulder at 3070 cm−1, assigned to ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), together with another δ(C
C), together with another δ(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) stretching band at 1635 cm−1 suggests the presence of a C
C) stretching band at 1635 cm−1 suggests the presence of a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond. Significant changes involve the [Al–H] site, 2a, which exhibits, according to DFT (but not observed experimentally because it is hidden in the
C double bond. Significant changes involve the [Al–H] site, 2a, which exhibits, according to DFT (but not observed experimentally because it is hidden in the ![[triple bond, length half m-dash]](https://www.rsc.org/images/entities/char_e007.gif) Si–O–Si
Si–O–Si![[triple bond, length half m-dash]](https://www.rsc.org/images/entities/char_e007.gif) combination and overtone bands at 1639, 1864, 1973 cm−1), a red-shift from ν(Al–H) = 1947 cm−1 to 1893 cm−1 (Fig. S5, ESI†). Further, a shift of (
combination and overtone bands at 1639, 1864, 1973 cm−1), a red-shift from ν(Al–H) = 1947 cm−1 to 1893 cm−1 (Fig. S5, ESI†). Further, a shift of (![[triple bond, length half m-dash]](https://www.rsc.org/images/entities/char_e007.gif) Si–H) from ν(Si–H) = 2256 cm−1 to 2225 cm−1 and (
Si–H) from ν(Si–H) = 2256 cm−1 to 2225 cm−1 and (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Si–H2) ν(SiH2) from 2191 cm−1 to 2144 cm−1 was observed, which is in accordance with the predicted IR spectrum from DFT calculations (Fig. S5, ESI†). A detailed explanation is given in the ESI.†
Si–H2) ν(SiH2) from 2191 cm−1 to 2144 cm−1 was observed, which is in accordance with the predicted IR spectrum from DFT calculations (Fig. S5, ESI†). A detailed explanation is given in the ESI.†
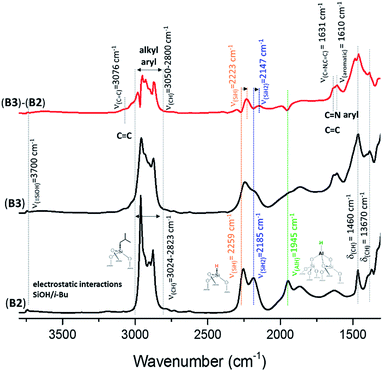 | ||
| Fig. 3 FT-IR spectra of Al–H@KCC-1700 (B2) and B3 formed after reaction with HG-II. Subtraction (B3 − B2) is given in red. | ||
After completion of the reaction, no gases (propane, propylene, or HCl) were released (ESI†). Elemental analysis (ESI†) shows the presence of 1.5 wt% of ruthenium on SBA15 (A3) and on KCC-1 (B3, dpore = 8 nm) corresponding to a Ru/Al-ratio of 0.1 and hence to a consumption of 10–15% of the available Al–H sites. The results are in agreement with energy dispersive X-ray (EDS) spectra measured by TEM for the regions shown in Fig. 6b and g where we found a Ru/Al ratio of 0.05 (Tables S4 and S5, ESI†). The partial and low consumption of Al–H sites might be due to steric hindrance induced by the immobilization of the bulky Ru complex (dHG-II ∼ 15 Å). For C3 (dpore = 4 nm), the amount of Ru was quantified to be 0.8 wt% corresponding to a consumption of only 7% of the available Al–H (ESI†), suggesting that Al–H sites are less accessible on this support. Investigating the Ru, Cl and N content relative to each other, we found that the Cl/Ru and N/Ru ratios remain at 2 as in the case of HG-II. These results, together with FT-IR results (no HCl detected),38 further prove that no HClg nor HClads was released during the grafting, suggesting that the structure of the catalysts with their two chlorines is maintained.
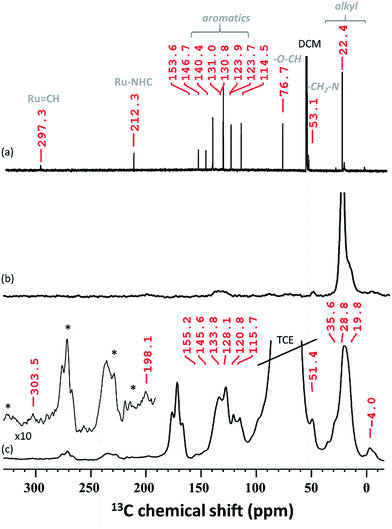
In solid-state NMR (SS NMR) spectroscopy, 1H and 13C signals indicate that the main functionalities of A3 (Fig. 4b and S6, ESI†) have been incorporated into the material. Signals at around 7 ppm (1H) and 135 ppm (13C) provide evidence that aromatic functionalities are still present. Furthermore, signals at 0.9 ppm (1H) and 23 ppm (13C) are detected and mainly attributed to the alkyl residues on the surface. To improve on the sensitivity levels of conventional SS NMR, dynamic nuclear polarization surface enhanced NMR spectroscopy (DNP SENS)39,40 of A3 has been performed. After establishing contact between A3 and TEKPol (16 mM TEKPol in tetrachloroethane (TCE)), we were able to detect a high 1H solvent enhancement (εH, defines the gain in intensity when comparing the solvent signal intensities of the microwave on/off spectra, ESI†) of 104, indicating that the radical was not destroyed as previously reported.41 DNP SENS analysis suggests that the carbene remains intact: a 13C signal at 303.5 ppm was obtained after 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 scans. Furthermore, a signal was found at around 198 ppm, which was assigned to Ru–CNHC.
000 scans. Furthermore, a signal was found at around 198 ppm, which was assigned to Ru–CNHC.
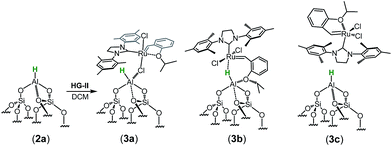
The spectrum obtained for the homogeneous analogue HG-II (Fig. 4a) shows a resonance for Ru![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH at 297 ppm and for the Ru–CNHC signal at 211 ppm. Furthermore, the aromatic signals, as well as the alkyl signals, are in accordance with those of HG-II. To figure out which functional group of HG-II (iso-propoxyl, chloride or tertiary amine ligands) preferentially interacts with the [Al–H] surface groups, we performed DFT calculations (Scheme 2) assuming 3 possibilities, 3a, 3b and 3c.
CH at 297 ppm and for the Ru–CNHC signal at 211 ppm. Furthermore, the aromatic signals, as well as the alkyl signals, are in accordance with those of HG-II. To figure out which functional group of HG-II (iso-propoxyl, chloride or tertiary amine ligands) preferentially interacts with the [Al–H] surface groups, we performed DFT calculations (Scheme 2) assuming 3 possibilities, 3a, 3b and 3c.
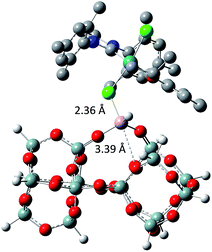
The tertiary amine in the N-heterocyclic carbene (NHC) ligand is not accessible for reaction with Al–H due to the bulkiness of the mesityl groups (3c in Scheme 2). The iso-propoxy-O lone pair preferentially maintains the interaction with the [Ru]-center rather than coordinating to the Al-center (3b in Scheme 2) by 0.7 kcal mol−1. Our DFT results show that only the lone pair of the chlorine is able to interact with the Al-center releasing 3.9 kcal mol−1 (3c in Scheme 2). The lone pair of the chloride coordinates to the Al-centre (Al⋯Cl–Ru) which leads simultaneously (similarly to the pyridine coordination) to the opening of the Al⋯O–Si-interaction from 2.02 Å to 3.39 Å (Scheme 3).
The catalytic performance of the three supported catalysts A3, B3 and C3 was evaluated in RCM of diethyl-diallyl malonate (DEDAM). Unfortunately, immobilized catalysts A3, B3 and C3 are not yet compatible for reactions with functionalized olefins, as diethyldiallyl malonate (DEDAM). RCM experiments of A3 in DCM showed (ESI†) that the catalyst leaches from the support (Fig. S12, ESI†). Leaching from A3 is reduced using toluene (low polarity) as the solvent, because the catalyst is less soluble and remains partially confined inside the channels of SBA15, as previously described.26,27,42
The leaching process leads to homogeneous HG-II which maintains its catalytic activity. Therefore, we conclude that the adsorption of HG-II is a reversible process, which is expected from DFT calculations.
To study the interactions of Al–H surface groups with DEDAM and the reason for the leaching, we performed DFT calculations. DFT results show that the interactions of the DEDAM carbonyl-oxygen with the Al-center are indeed thermodynamically favoured (−1.8 kcal mol−1) but 2.1 kcal mol−1 less than those with HG-II. However, these findings might explain a competition between both interactions leading to the leaching of [Ru] (ESI†).
In parallel, we investigated A3, B3 and C3, together with the homogeneous analogue catalyst HG-II in propene metathesis in a continuous flow reactor (Fig. 5). The activity of immobilized catalysts A3, B3 and C3 in propene metathesis is improved in comparison to the homogeneous system by a factor of 2 (A3), 3 (C3) and even 5 for B3. Such an improvement was not observed by Sels et al.42 in liquid phase cyclooctene metathesis.
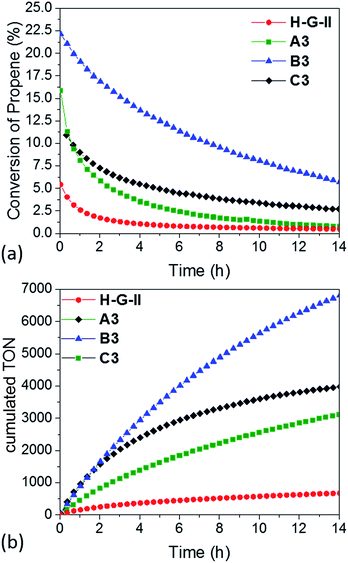 | ||
| Fig. 5 (a) Conversion and (b) cumulative TON of propene-metathesis (propene: 16 mL min−1; T = 25 °C, [Ru]: 9 μmol) over HG-II (red), A3 (green), B3 (blue) and C3 (black). | ||
The maximum conversion in dynamic gas phase reaction was 5% for HG-II, 11% for A3, 15% for C3 and 22% for B3. The cumulative TONs after 14 hours of reaction increase from 677 (HG-II) to 3113 (A3), 3976 (C3), and 6807 (B3). The improved activity might be explained by a decreased electron density on the metal centre due to the (Al⋯Cl–Ru) interaction. Electronic tuning of the anionic ligands of 2nd generation Ru olefin metathesis complexes is known to change the catalytic activity of the catalyst.9,43–45 The activity of B3 is enhanced compared to that of A3 and C3 because active sites are more accessible as they reside at the external surface of KCC-1 (B3).
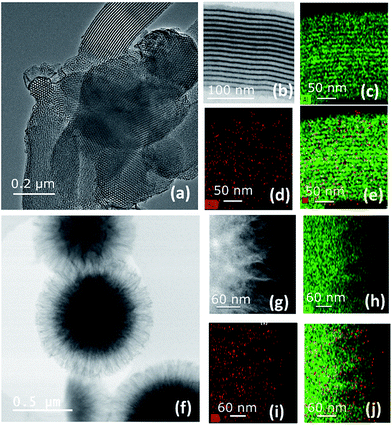
To gain better understanding of the distribution of HG-II and its accessibility inside the mesopores of SBA15 and KCC-1 of A3 and B3, we performed transmission electron microscopy (TEM) analyses (Fig. 6). Moreover, a double-aberration corrected TEM of model Titan ThemisZ from Thermo Fisher Scientific was employed to complete the mentioned analysis. It has to be noted that for each mesoporous sample, a dry specimen preparation was adapted whereby a modicum of specimen was placed onto holey-carbon coated copper grids. Spherical aberration-corrected bright-field TEM (BF-TEM) images of several particles were acquired and revealed that the structure of both materials was maintained (Fig. 6a and f). Elemental distributions of Ru and Al in both samples were determined by using the STEM-EDS spectrum imaging technique. These elemental maps contain a high degree of confidence in regard to revealing the presence of Ru and Al as these are generated by acquiring the EDS signal with a high solid-angle EDS detector of model SuperX. The elemental maps of Al are shown in Fig. 6c and h, and those of Ru in Fig. 6d and i. The superimposed Al and Ru maps are shown in Fig. 6e and j. More detailed information about EDS experiments can be found in the ESI.†
Two main pieces of information are obtained from the EF-TEM image comparing A3 (SBA15, dpore = 6 nm, Fig. 6a–e) and B3 (KCC-1, dpore = 8 nm, Fig. 6f–j). The first one is that [Ru] (red) is well-distributed on both mesoporous materials (Al = green) (Fig. 6e and j). The second one highlights a partial obstruction at the pore openings leading to the low loading of active sites at the center of the hexagonal mesopores. This explains the poor catalytic results obtained in propene metathesis, which are better using KCC-1 as a support as the active sites are more accessible.
3 Conclusions
The aim of this work was to investigate the type of interaction occurring between the 2nd generation Hoveyda–Grubbs catalyst HG-II and two types of Al-modified mesoporous silica, SBA15 and KCC-1, synthesized through the SOMC concept, strategy and methodology. These mesoporous supports feature well-defined tetrahedral aluminum hydride sites having a strong Lewis acid character, [(![[triple bond, length half m-dash]](https://www.rsc.org/images/entities/char_e007.gif) Si–O–Si
Si–O–Si![[triple bond, length half m-dash]](https://www.rsc.org/images/entities/char_e007.gif) )(
)(![[triple bond, length half m-dash]](https://www.rsc.org/images/entities/char_e007.gif) Si–O–)2Al–H]. Therefore, the immobilization of HG-II occurs through Lewis acid–base interactions as evidenced by gas phase analysis, FTIR, elemental analysis, SS NMR, DNP SENS and DFT calculations. The catalytic activity of all the materials was tested in propene metathesis. An increased activity of immobilized catalysts A3, B3 and C3 compared to their HG-II analogue arises from the Al⋯Cl–Ru interaction, making the [Ru]-center more electropositive and hence more reactive. Also the type of support affects the catalytic results. While A3 (SBA15) is beneficial for trapping and protecting the catalyst inside the mesopores (DNP SENS, leaching), the accessibility of the active sites is reduced (lower TONs). In contrast, B3 and C3 (KCC-1) are more active in propene metathesis, because the active sites reside on the external surface and are hence fully accessible to the substrate.
Si–O–)2Al–H]. Therefore, the immobilization of HG-II occurs through Lewis acid–base interactions as evidenced by gas phase analysis, FTIR, elemental analysis, SS NMR, DNP SENS and DFT calculations. The catalytic activity of all the materials was tested in propene metathesis. An increased activity of immobilized catalysts A3, B3 and C3 compared to their HG-II analogue arises from the Al⋯Cl–Ru interaction, making the [Ru]-center more electropositive and hence more reactive. Also the type of support affects the catalytic results. While A3 (SBA15) is beneficial for trapping and protecting the catalyst inside the mesopores (DNP SENS, leaching), the accessibility of the active sites is reduced (lower TONs). In contrast, B3 and C3 (KCC-1) are more active in propene metathesis, because the active sites reside on the external surface and are hence fully accessible to the substrate.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The research reported in this publication was supported by funding from King Abdullah University of Science and Technology (KAUST). The authors are grateful to the KAUST Supercomputing Laboratory (KSL) for the resources provided.References
- G. C. Vougioukalakis and R. H. Grubbs, Chem. Rev., 2010, 110, 1746–1787 CrossRef CAS PubMed.
- T. P. Montgomery, A. M. Johns and R. H. Grubbs, Catalysts, 2017, 7, 87 CrossRef.
- M. B. Herbert and R. H. Grubbs, Angew. Chem., Int. Ed., 2015, 54, 5018–5024 CrossRef CAS PubMed.
- S. P. Nolan and H. Clavier, Chem. Soc. Rev., 2010, 39, 3305–3316 RSC.
- C. E. Diesendruck, E. Tzur and N. G. Lemcoff, Eur. J. Inorg. Chem., 2009, 4185–4203 CrossRef CAS.
- S. B. Garber, J. S. Kingsbury, B. L. Gray and A. H. Hoveyda, J. Am. Chem. Soc., 2000, 122, 8168–8179 CrossRef CAS.
- C. Samojlowicz, M. Bieniek and K. Grela, Chem. Rev., 2009, 109, 3708–3742 CrossRef CAS PubMed.
- R. H. Grubbs, Angew. Chem., Int. Ed., 2006, 45, 3760–3765 CrossRef CAS PubMed.
- T. M. Trnka and R. H. Grubbs, Acc. Chem. Res., 2001, 34, 18–29 CrossRef CAS PubMed.
- R. H. Grubbs, J. Macromol. Sci., Part A: Pure Appl.Chem., 1994, 31, 1829–1933 CrossRef.
- O. Nuyken and S. D. Pask, Polymers, 2013, 5, 361–403 CrossRef.
- A. R. Hlil, S. Moncho, R. Tuba, K. Elsaid, G. Szarka, E. N. Brothers, R. H. Grubbs, M. Al-Hashimi and H. S. Bazzi, J. Catal., 2016, 344, 100–107 CrossRef CAS.
- H.-K. Lee, K.-T. Bang, A. Hess, R. H. Grubbs and T.-L. Choi, J. Am. Chem. Soc., 2015, 137, 9262–9265 CrossRef CAS PubMed.
- R. H. Grubbs, S. J. Miller and G. C. Fu, Acc. Chem. Res., 1995, 28, 446–452 CrossRef CAS.
- T. S. Ahmed and R. H. Grubbs, J. Am. Chem. Soc., 2017, 139, 1532–1537 CrossRef CAS PubMed.
- M. S. Sanford, J. A. Love and R. H. Grubbs, J. Am. Chem. Soc., 2001, 123, 6543–6554 CrossRef CAS PubMed.
- J. C. Conrad and D. E. Fogg, Curr. Org. Chem., 2006, 10, 185–202 CAS.
- G. Szczepaniak, K. Kosinski and K. Grela, Green Chem., 2014, 16, 4474–4492 RSC.
- H. Balcar and J. Čejka, Coord. Chem. Rev., 2013, 257, 3107–3124 CrossRef CAS.
- H. Balcar and J. Čejka, Macromol. Symp., 2010, 293, 43–47 CrossRef CAS.
- M. P. Conley, C. Copéret and C. Thieuleux, ACS Catal., 2014, 4, 1458–1469 CrossRef CAS.
- C. Copéret and J. M. Basset, Adv. Synth. Catal., 2007, 349, 78–92 CrossRef.
- D. Bek, H. Balcar, N. Žilková, A. Zukal, M. Horáček and J. Čejka, ACS Catal., 2011, 1, 709–718 CrossRef CAS.
- H. Zhang, Y. Li, S. Shao, H. Wu and P. Wu, J. Mol. Catal. A: Chem., 2013, 372, 35–43 CrossRef CAS.
- J. Pastva, K. Skowerski, S. J. Czarnocki, N. Žilková, J. Čejka, Z. Bastl and H. Balcar, ACS Catal., 2014, 4, 3227–3236 CrossRef CAS.
- H. Yang, Z. Ma, Y. Wang, Y. Wang and L. Fang, Chem. Commun., 2010, 46, 8659–8661 RSC.
- B. Van Berlo, K. Houthoofd, B. F. Sels and P. A. Jacobs, Adv. Synth. Catal., 2008, 350, 1949–1953 CrossRef CAS.
- M. Bru, R. Dehn, J. H. Teles, S. Deuerlein, M. Danz, I. B. Müller and M. Limbach, Chem.–Eur. J., 2013, 19, 11661–11671 CrossRef CAS PubMed.
- J. D. A. Pelletier and J.-M. Basset, Acc. Chem. Res., 2016, 49, 664–677 CrossRef CAS PubMed.
- C. Copéret, A. Comas-Vives, M. P. Conley, D. P. Estes, A. Fedorov, V. Mougel, H. Nagae, F. Núñez-Zarur and P. A. Zhizhko, Chem. Rev., 2016, 116, 323–421 CrossRef PubMed.
- A. Bendjeriou-Sedjerari, J. M. Azzi, E. Abou-Hamad, D. H. Anjum, F. A. Pasha, K.-W. Huang, L. Emsley and J.-M. Basset, J. Am. Chem. Soc., 2013, 135, 17943–17951 CrossRef CAS PubMed.
- A. Bendjeriou-Sedjerari, J. Sofack-Kreutzer, Y. Minenkov, E. Abou-Hamad, B. Hamzaoui, B. Werghi, D. H. Anjum, L. Cavallo, K.-W. Huang and J.-M. Basset, Angew. Chem., Int. Ed., 2016, 55, 11162–11166 CrossRef CAS PubMed.
- B. Werghi, A. Bendjeriou-Sedjerari, J. Sofack-Kreutzer, A. Jedidi, E. Abou-Hamad, L. Cavallo and J.-M. Basset, Chem. Sci., 2015, 6, 5456–5465 RSC.
- B. Werghi, A. Bendjeriou-Sedjerari, A. Jedidi, E. Abou-Hamad, L. Cavallo and J.-M. Basset, Organometallics, 2016, 35, 3288–3294 CrossRef CAS.
- D. Zhao, J. Feng, Q. Huo, N. Melosh, G. H. Fredrickson, B. F. Chmelka and G. D. Stucky, Science, 1998, 279, 548–552 CrossRef CAS PubMed.
- V. Polshettiwar, D. Cha, X. Zhang and J. M. Basset, Angew. Chem., Int. Ed., 2010, 49, 9652–9656 CrossRef CAS PubMed.
- N. Bayal, B. Singh, R. Singh and V. Polshettiwar, Sci. Rep., 2016, 6, 24888 CrossRef CAS PubMed.
- C. Paze, B. Civalleri, S. Bordiga and A. Zecchina, J. Phys. Chem. B, 1998, 102, 10753–10764 CrossRef CAS.
- E. Pump, J. Viger-Gravel, E. Abou-Hamad, M. K. Samantaray, B. Hamzaoui, A. Gurinov, D. H. Anjum, D. Gajan, A. Lesage, A. Bendjeriou-Sedjerari, L. Emsley and J.-M. Basset, Chem. Sci., 2017, 8, 284–290 RSC.
- D. Lee, H. Takahashi, A. S. L. Thankamony, J.-P. Dacquin, M. Bardet, O. Lafon and G. D. Paëpe, J. Am. Chem. Soc., 2012, 134, 18491–18494 CrossRef CAS PubMed.
- M. K. Samantaray, J. Alauzun, D. Gajan, S. Kavitake, A. Mehdi, L. Veyre, M. Lelli, A. Lesage, L. Emsley, C. Copéret and C. Thieuleux, J. Am. Chem. Soc., 2013, 135, 3193–3199 CrossRef CAS PubMed.
- A. Dewaele, B. Van Berlo, J. Dijkmans, P. A. Jacobs and B. F. Sels, Catal. Sci. Technol., 2016, 6, 2580–2597 CAS.
- J. Wappel, C. A. Urbina-Blanco, M. Abbas, J. H. Albering, R. Saf, S. P. Nolan and C. Slugovc, Beilstein J. Org. Chem., 2010, 6, 1091–1098 CrossRef CAS PubMed.
- S. Guidone, O. Songis, L. Falivene, F. Nahra, A. M. Z. Slawin, H. Jacobsen, L. Cavallo and C. S. J. Cazin, ACS Catal., 2015, 5, 3932–3939 CrossRef CAS.
- E. L. Dias, S. T. Nguyen and R. H. Grubbs, J. Am. Chem. Soc., 1997, 119, 3887–3897 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7sc05200f |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2018 |