Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Diastereodivergent asymmetric Michael-alkylation reactions using chiral N,N′-dioxide/metal complexes†
Yulong
Kuang‡
 a,
Bin
Shen‡
a,
Bin
Shen‡
 a,
Li
Dai
a,
Li
Dai
 a,
Qian
Yao
a,
Qian
Yao
 a,
Xiaohua
Liu
a,
Xiaohua
Liu
 *a,
Lili
Lin
*a,
Lili
Lin
 a and
Xiaoming
Feng
a and
Xiaoming
Feng
 *ab
*ab
aKey Laboratory of Green Chemistry & Technology, Ministry of Education, College of Chemistry, Sichuan University, Chengdu 610064, China. E-mail: liuxh@scu.edu.cn; xmfeng@scu.edu.cn; Fax: +86 28 85418249; Tel: +86 28 85418249
bCollaborative Innovation Center of Chemical Science and Engineering (Tianjin), P. R. China
First published on 8th November 2017
Abstract
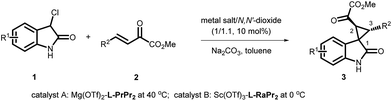
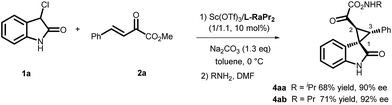
A diastereodivergent asymmetric Michael-alkylation reaction between 3-chloro-oxindoles and β,γ-unsaturated-α-ketoesters has been achieved using L-RaPr2/Sc(OTf)3 and L-PrPr2/Mg(OTf)2 metal complexes as catalysts. Both rel-(1R,2S,3R) and rel-(1S,2S,3R) chiral spiro cyclopropane oxindoles were constructed in good yields, diastereoselectivities and ee values. The diastereodivergent control may originate from different alkylation pathways after the Michael addition, with either intramolecular trapping of the aza-ortho-xylylene intermediate or direct SN2 substitution.
Tuning diastereoselectivity in catalytic asymmetric synthesis is challenging due to the inherent preference for forming one type of diastereomer in most reactions.1 However, relative configurations are as important as absolute configurations in pharmacology and drug discovery because both can influence the physiological activity of a molecule.2 In general, diastereodivergence3 can be realized by changing the catalyst,4 additive and solvent,5 substrate,6 and other methods. Nevertheless, diastereodivergent synthesis is still in its infancy. It’s desirable to develop new strategies and discover more diastereodivergent reactions.
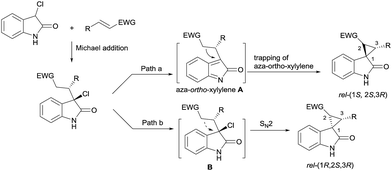
Oxindoles with a unique spirocyclopropane moiety exhibit diverse biological activities, such as non-nucleoside reverse transcriptase inhibitor and antitumor activity.7 Among the synthetic methodologies to prepare these molecules,8 the cascade Michael-alkylation reactions9 of 3-chlorooxindole with α,β-unsaturated olefins provide an operationally simple, stepwise pathway for diastereodivergence. Currently, only organic proline-based silyl ethers,10 cinchona alkaloid-derived thioureas11 and squaramide12 catalysts have been developed. These reactions proceed via the intramolecular trapping of the chiral aza-ortho-xylylene intermediate, A, after the Michael addition to afford the thermodynamically favored rel-(1S,2S,3R)13 products (Scheme 1, path a). On the other hand, if the Michael addition products follow the direct SN2 substitution pathway (intermediate B), the rel-(1R,2S,3R) products will be formed (Scheme 1, path b). To the best of our knowledge, there is no precedent for synthesizing the rel-(1R,2S,3R) products as the major diastereomer, much less synthesizing both rel-(1S,2S,3R) and rel-(1R,2S,3R) products in high efficiency without drastically changing the reaction conditions. Herein, we reported a diastereodivergent asymmetric Michael-alkylation reaction between 3-chloro-oxindoles and β,γ-unsaturated-α-ketoesters using chiral N,N′-dioxide/metal complexes,14 synthesizing rel-(1S,2S,3R) and rel-(1R,2S,3R) spirocyclopropane oxindoles in high yields, diastereoselectivities and enantioselectivities.
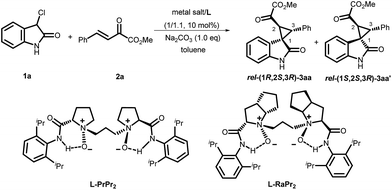
Initially, the cascade reaction of 3-Cl oxindole, 1a, with the β,γ-unsaturated-α-ketoester 2a was chosen as the model reaction to optimize the reaction conditions. First, a series of metal salts were investigated by complexing with chiral N,N′-dioxide L-PrPr2 in the presence of Na2CO3 as the base at 30 °C. It was found that both Sc(OTf)3 and Mg(OTf)2 complexes could catalyze the reaction with a preference for forming a different diastereomer. The enantioselectivity was moderate for each major diastereomer, which could be isolated by column chromatography. Next, other conditions were screened.15 The L-RaPr2/Sc(OTf)3 complex elevated the isolated yield of the product rel-(1R,2S,3R)-3aa to 92% with 78% ee (entry 3). After lowering the reaction temperature to 0 °C, increasing the stoichiometry of the base to 1.3 equivalents, and prolonging the reaction time to 72 h, the rel-(1R,2S,3R)-3aa could be obtained in 97% yield and 92% ee (entry 4, Table 1).
| Entry | Metal salt | L* | Yieldb (3aa/3aa′) (%) | eec (3aa/3aa′) |
|---|---|---|---|---|
a Unless otherwise noted, the reaction proceeded with 1a (0.1 mmol), 2a (0.1 mmol), metal salt/ligand (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1, 10 mol%), and Na2CO3 (1.0 equiv.) in toluene (1.0 mL) at 30 °C for 24 h.
b Isolated yield.
c Determined by chiral HPLC on a chiral stationary phase (Chiralcel IA and IE).
d The reaction proceeded at 0 °C for 72 h and with 1.3 eq. of Na2CO3.
e At 40 °C for 72 h. 1.1, 10 mol%), and Na2CO3 (1.0 equiv.) in toluene (1.0 mL) at 30 °C for 24 h.
b Isolated yield.
c Determined by chiral HPLC on a chiral stationary phase (Chiralcel IA and IE).
d The reaction proceeded at 0 °C for 72 h and with 1.3 eq. of Na2CO3.
e At 40 °C for 72 h.
|
||||
| 1 | Sc(OTf)3 | L-PrPr 2 | 92/7 | 76/0 |
| 2 | Mg(OTf)2 | L-PrPr 2 | 14/28 | 0/88 |
| 3 | Sc(OTf)3 | L-RaPr 2 | 92/— | 78/— |
| 4d | Sc(OTf)3 | L-RaPr 2 | 97/— | 92/— |
| 5e | Mg(OTf)2 | L-PrPr 2 | —/77 | —/95 |
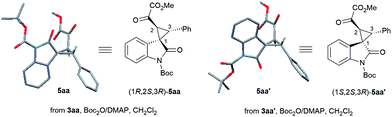
On the other hand, using chiral L-PrPr2/Mg(OTf)2 as the catalyst and increasing the reaction temperature to 40 °C, the corresponding rel-(1S,2S,3R)-3aa′ could be afforded in 77% yield and 95% ee after 72 hours (entry 5). It is worth mentioning that the metal cations dictate the diastereoselectivity and variation of the chiral ligand structure and the reaction temperature did not change the major diastereoisomer once Sc(OTf)3 or Mg(OTf)2 were identified as suitable catalysts. The absolute configurations of the major enantiomers were determined separately by X-ray crystallographic analysis of the corresponding N-Boc protected derivatives 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 (Fig. 1). Through this method, rel-(1S,2S,3R)-5aa′ obtained from the L-PrPr2/Mg(OTf)2 catalytic system was determined to be (1S, 2S, 3R), and rel-(1R,2S,3R)-5aa generated from L-RaPr2/Sc(OTf)3 was found to be (1R, 2S, 3R).
16 (Fig. 1). Through this method, rel-(1S,2S,3R)-5aa′ obtained from the L-PrPr2/Mg(OTf)2 catalytic system was determined to be (1S, 2S, 3R), and rel-(1R,2S,3R)-5aa generated from L-RaPr2/Sc(OTf)3 was found to be (1R, 2S, 3R).
Next, the enantioselective and diastereodivergent synthesis of a series of spirocyclopropane oxindoles was carried out using these two chiral catalyst systems (Table 2). Under the optimized conditions, all of the substrates gave one major diastereomer (higher than 94:6 diastereoselectivity) with moderate to excellent yields and enantioselectivities. It is worth noting that most of the rel-(1R,2S,3R) products 3 were unstable under the HPLC analysis conditions. Fortunately, the enantiomeric excess could be determined after conversion of rel-(1R,2S,3R)-3 into the corresponding derivatives rel-(1R,2S,3R)-4 (Scheme 2). Generally, the rel-(1S,2S,3R)-diastereoisomers prepared from the L-PrPr2/Mg(OTf)2 catalyst were delivered in higher enantioselectivities than the rel-(1R,2S,3R) isomers from the L-RaPr2/Sc(OTf)3 catalyst. For the synthesis of rel-(1S,2S,3R) cyclopropanes, 3-chlorooxindoles 1 with halo-substituents at the C4 and C5-positions gave higher enantioselectivities than C6-substituted ones (entries 3–8). Electron-donating or -withdrawing substituents on the aromatic β,γ-unsaturated-α-ketoester 2 had a slight influence on the enantioselectivity (entries 10–22). 2-Naphthyl- and 2-thiophenyl substituted β,γ-unsaturated-α-ketoesters were also tolerated well (entries 21 and 22). Moreover, when the aliphatic substrates 2t and 2u were subjected to the reaction conditions, the corresponding rel-(1R,2S,3R)-products could be obtained in excellent yields with excellent enantioselectivities (up to 99% yield and 99% ee; entries 23 and 24).
| Entry | 3: R1; R2 | rel-(1S,2S,3R)-3′ (Cat A) | rel-(1R,2S,3R)-3 (Cat B) | ||
|---|---|---|---|---|---|
| Yieldb (%) | eec (%) | Yieldb (%) | eed (%) | ||
| a Unless otherwise noted, reactions were performed with 1 (0.1 mmol), 2 (0.1 mmol), chiral catalyst (10 mol%) and Na2CO3 in toluene (1.0 mL) for 72 h. For rel-(1S,2S,3R)-3′, L-PrPr2/Mg(OTf)2 (1.1/1) and 1.0 eq. of Na2CO3 were used at 40 °C. For rel-(1R,2S,3R)-3, L-RaPr2/Sc(OTf)3 (1.1/1, 10 mol%) and 1.3 eq. of Na2CO3 were used at 0 °C. b Isolated yield. c Determined by HPLC on a chiral stationary phase. d Determined by HPLC on a chiral stationary phase after transformation into 4. e The diastereoselectivity of 3ah was determined to be 94:6 from HPLC. | |||||
| 1 | 3aa(′): H; C6H5 | 77 | 95 | 97 | 92 |
| 2 | 3ba(′): 4-Me; C6H5 | — | — | 67 | 86 |
| 3 | 3ca(′): 4-F; C6H5 | 84 | 96 | 76 | 86 |
| 4 | 3da(′): 5-Me; C6H5 | 72 | 96 | 96 | 89 |
| 5 | 3ea(′): 5-F; C6H5 | 67 | 92 | 96 | 81 |
| 6 | 3fa(′): 5-Cl; C6H5 | 61 | 88 | — | — |
| 7 | 3ga(′): 6-F; C6H5 | 70 | 89 | 91 | 90 |
| 8 | 3ha(′): 6-Cl; C6H5 | 63 | 84 | 98 | 88 |
| 9 | 3ia(′): 6-Br; C6H5 | — | — | 83 | 84 |
| 10 | 3ah(′): H; 2-MeOC6H4 | 69e | 95e | 71 | 91 |
| 11 | 3ai(′): H; 3-MeOC6H4 | 70 | 96 | 98 | 95 |
| 12 | 3aj(′): H; 4-MeOC6H4 | 59 | 94 | 96 | 72 |
| 13 | 3ak(′): H; 2-MeC6H4 | 50 | 91 | 76 | 96 |
| 14 | 3al(′): H; 4-MeC6H4 | 77 | 93 | 93 | 87 |
| 15 | 3av(′): H; 4-FC6H4 | — | — | 96 | 90 |
| 16 | 3am(′): H; 3-ClC6H4 | 77 | 92 | 76 | 87 |
| 17 | 3an(′): H; 4-ClC6H4 | 63 | 91 | 79 | 93 |
| 18 | 3ao(′): H; 2-BrC6H4 | 62 | 94 | — | — |
| 19 | 3ap(′): H; 4-BrC6H4 | 81 | 94 | 83 | 94 |
| 20 | 3aq(′): H; 4-PhC6H4 | 74 | 98 | 96 | 92 |
| 21 |
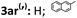
|
71 | 93 | 91 | 93 |
| 22 |
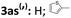
|
52 | 95 | — | — |
| 23 | 3at(′): H; nBu | 71 | 93 | 95 | 99 |
| 24 | 3au(′): H; cyclohexyl | — | — | 99 | 99 |
Preliminary mechanistic studies were conducted to confirm our proposed diastereodivergent control mode. The relationship between the ee values of the ligand and product showed a linear correlation.15 Additionally, X-ray crystallographic analysis of the catalysts also showed a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of ligand to metal.17 Both of these findings imply that the monomeric catalysts might be the main catalytically active species. What’s more, crystal structures of both catalysts displayed similar geometries and showed no significant differences in the accessibility of the substrate’s coordination site. To check whether L-PrPr2/Mg(OTf)2 and L-RaPr2/Sc(OTf)3 coordinated with different substrates, in situ HRMS analysis was performed.15 Based on the HRMS spectra, both of them coordinate with the same substrate, 3-Cl oxindole, to initiate the reaction. All of these experiments excluded the possibility that the observed diastereodivergence resulted from different coordinative styles of the two catalysts.
1 ratio of ligand to metal.17 Both of these findings imply that the monomeric catalysts might be the main catalytically active species. What’s more, crystal structures of both catalysts displayed similar geometries and showed no significant differences in the accessibility of the substrate’s coordination site. To check whether L-PrPr2/Mg(OTf)2 and L-RaPr2/Sc(OTf)3 coordinated with different substrates, in situ HRMS analysis was performed.15 Based on the HRMS spectra, both of them coordinate with the same substrate, 3-Cl oxindole, to initiate the reaction. All of these experiments excluded the possibility that the observed diastereodivergence resulted from different coordinative styles of the two catalysts.
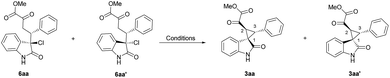
The intermediates of the Michael addition products were then synthesized and subjected to the optimized reaction conditions. The relative configuration of 6aa was also confirmed by X-ray crystallographic analysis.18 As summarized in Table 3, the two diastereomers of 6 only transformed to the same 3aa′ with 76% yield and 31% yield, respectively, under the L-PrPr2/Mg(OTf)2 system (Table 3, entries 2 and 4), which may be due to the chiral match or mismatch effect. On the contrary, 3aa or 3aa′ could be afforded from either 6aa or 6aa′ in the L-RaPr2/Sc(OTf)3 system with 18% yield and 74% yield, respectively (Table 3, entries 1 and 3). By comparing the different results between the L-RaPr2/Sc(OTf)3 and L-PrPr2/Mg(OTf)2 catalyzed reactions, L-PrPr2/Mg(OTf)2 should promote this reaction with aza-ortho-xylylene intermediates, as has been reported previously, which induced thermodynamically favored rel-(1S,2S,3R) 3aa′ from different diastereomers of 6. However, in the L-RaPr2/Sc(OTf)3 catalytic system, the chirality inversion at the quaternary carbon from 6aa to rel-(1R,2S,3R) 3aa and from 6aa′ to rel-(1S,2S,3R) 3aa′ illustrated that alkylation proceeded through a direct SN2 substitution pathway. Moreover, chiral 6aa (−73% ee) could produce another enantiomer of 3aa′ in 75% yield and 76% ee under the L-PrPr2/Mg(OTf)2 conditions (Table 3, entry 5). Undoubtedly, the same enantioselectivity revealed that the first Michael addition step should be the chirality-determining step. For the result of entry 1, the product 3aa was formed with 85% ee and the starting material was recovered in 80% yield with 84:16 diastereoselectivity (6aa:6aa′) from racemic 6aa under the L-RaPr2/Sc(OTf)3 catalytic system (Table 3, entry 1). We propose that this result came from the chemical equilibrium between the retro-Michael reaction and Michael addition, which could also account for the lack of formation of rel-(1S,2S,3R) 3aa′. The low yield also implied that the rate of SN2 substitution from the enolate ion is high and Na2CO3 is not a strong enough base to deprotonate the Michael intermediate. To evaluate the role of the N–H group, N-methylated 3-Cl oxindole was also subjected into the reaction, which showed a poor result.15
| Entry | 6 | Conditiona | 3aa [yield, ee] | 3aa′ [yield, ee] |
|---|---|---|---|---|
| a Unless otherwise noted, reactions were performed with corresponding 6 and catalyst (M/L = 1/1.1 10 mol%) and Na2CO3 (1.0 eq.) in toluene (1.0 mL) at 0 °C for 72 h. b Isolated yield. ee was determined by HPLC on a chiral stationary phase. c The recovered 6aa had a yield of 80%, 84:16 dr, and −17% ee/19% ee, and the diastereoselectivity of corresponding 3aa was 84:16. d The ee of 6aa was −73% ee. | ||||
| 1c | (±) 6aa | L-RaPr2/Sc(OTf)3 | 18%, 85% | — |
| 2 | (±) 6aa | L-PrPr2/Mg(OTf)2 | — | 76%, −7% |
| 3 | (±) 6aa′ | L-RaPr2/Sc(OTf)3 | — | 74%, 3% |
| 4 | (±) 6aa′ | L-PrPr2/Mg(OTf)2 | — | 31%, race |
| 5d | (+) 6aa | L-PrPr2/Mg(OTf)2 | — | 75%, −76% |
Conclusions
A diastereodivergent asymmetric Michael-alkylation reaction between 3-Cl oxindoles and β,γ-unsaturated-α-ketoesters was accomplished by tuning metal catalysts and adjusting the ligands and temperature. Under the optimized conditions, both rel-(1R,2S,3R) and rel-(1S,2S,3R) spiro cyclopropane oxindoles were synthesized with high yields, diastereoselectivities and enantioselectivities. Mechanistic studies also revealed that the diastereodivergent control should come from either trapping the aza-ortho-xylylene intermediates or direct SN2 substitution in the alkylation step, which may be caused by the different characteristics of the metal catalysts. Developing other diastereodivergent asymmetric methodologies with this strategy is ongoing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the National Natural Science Foundation of China (21432006, 21290182 and 21772127) for financial support.Notes and references
- E. M. Carreira and L. Kvaerno, Classics in Stereoselective Synthesis, Wiley, Weinheim, 2009 Search PubMed.
- (a) C. Wolf, Dynamic stereochemistry of chiral compounds: principles and applications, Royal Society of Chemistry, 2008 Search PubMed; (b) C. G. Wermuth, The Practice of Medicinal Chemistry, Elsevier, Amsterdam, 3rd edn, 2008 Search PubMed; (c) W. O. Foye, Foye’s Principles of Medicinal Chemistry, Lippincott Williams & Wilkins, 7th edn, 2013 Search PubMed.
- Reviews in asymmetric diastereodivergent synthesis: (a) L. Lin and X. M. Feng, Chem.–Eur. J., 2017, 23, 6464 CrossRef CAS PubMed; (b) M. Bihani and J. C. G. Zhao, Adv. Synth. Catal., 2017, 359, 534 CrossRef CAS; (c) G. Zhan, W. Du and Y. C. Chen, Chem. Soc. Rev., 2017, 46, 1675 RSC.
- For selected examples of diastereodivergent asymmetric synthesis by changing the catalysis and dual catalyst strategies, see: (a) E. E. Maroto, S. Filippone, M. Suárez, R. Martínez-Álvarez, A. de Cozar, F. P. Cossío and N. Martín, J. Am. Chem. Soc., 2014, 136, 705 CrossRef CAS PubMed; (b) S. Krautwald, D. Sarlah, M. A. Schafroth and E. M. Carreira, Science, 2013, 340, 1065 CrossRef CAS PubMed; (c) M. T. Oliveira, M. Luparia, D. Audisio and N. Maulide, Angew. Chem., Int. Ed., 2013, 52, 13149 CrossRef CAS PubMed; (d) E. Conde, D. Bello, A. de Cózar, M. Sánchez, M. A. Vázquez and F. P. Cossío, Chem. Sci., 2012, 3, 1486 RSC; (e) B. Simmons, A. M. Walji and D. W. C. MacMillan, Angew. Chem., Int. Ed., 2009, 48, 4349 CrossRef CAS PubMed; (f) S. Filippone, E. E. Maroto, A. Martín-Domenech, M. Suarez and N. Martín, Nat. Chem., 2009, 1, 578 CrossRef CAS PubMed; (g) X. X. Yan, Q. Peng, Y. Zhang, K. Zhang, W. Hong, X. L. Hou and Y. D. Wu, Angew. Chem., Int. Ed., 2006, 45, 1979 CrossRef CAS PubMed; (h) Y. Huang, A. M. Walji, C. H. Larsen and D. W. C. MacMillan, J. Am. Chem. Soc., 2005, 127, 15051 CrossRef CAS PubMed.
- Selected examples: (a) X. Wu, Z. Chen, Y.-B. Bai and V. M. Dong, J. Am. Chem. Soc., 2016, 138, 12103 Search PubMed; (b) X. Li, M. Lu, Y. Dong, W. Wu, Q. Qian, J. Ye and D. J. Dixon, Nat. Commun., 2014, 5, 4479 CAS; (c) X. Tian, C. Cassani, Y. Liu, A. Moran, A. Urakawa, P. Galzerano, E. Arceo and P. Melchiorre, J. Am. Chem. Soc., 2011, 133, 17934 CrossRef CAS PubMed.
- J. Zhu, Y. Liang, L. Wang, Z. B. Zheng, K. N. Houk and Y. Tang, J. Am. Chem. Soc., 2014, 136, 6900 CrossRef CAS PubMed.
- (a) Y. He, T. Jiang, K. L. Kuhen, Y. H. Ellis, B. Wu, T. Y. H. Wu and B. Bursulaya, Oxindoles with Anti-HIV Activity, U.S. Pat., WO 2004/037247A1, 2004; (b) T. Jiang, K. L. Kuhen, K. Wolff, H. Yin, K. Bieza, J. Caldwell, B. Bursulaya, T. Y. H. Wu and Y. He, Bioorg. Med. Chem. Lett., 2006, 16, 2105 CrossRef CAS PubMed; (c) P. B. Sampson, Y. Liu, S. W. Li, B. T. Forrest, H. W. Pauls, L. G. Edwards, M. Feher, N. K. B. Patel, R. Laufer and G. Pan, Kinase Inhibitors and Method of Treating Cancer with Same, U.S. Pat., WO 2010/115279A1, 2010; (d) L. Chen, L. Feng, Y. He, M. Huang and H. Yun, Spiro Indole Cyclopropane Indolinones Useful as Ampk Modulators, U.S. Pat., WO2011/70039A1, 2011; (e) H. W. Pauls, S. W. Li, P. B. Sampson and B. T. Forrest, Plk-4 Inhibitors and Methods of Treating Cancer with Same, U.S. Pat., WO 2012/048411A1, 2012; (f) P. B. Sampson, Y. Liu and N. K. Patel, et al. , J. Med. Chem., 2015, 58, 130 CrossRef CAS PubMed; (g) P. B. Sampson, et al. , J. Med. Chem., 2015, 58, 147 CrossRef CAS PubMed.
- Selected examples: (a) Z. Y. Cao, F. Zhou, Y. H. Yu and J. Zhou, Org. Lett., 2013, 15, 42 CrossRef CAS PubMed; (b) Z. Y. Cao, X. M. Wang, C. Tan, X. L. Zhao, J. Zhou and K. Ding, J. Am. Chem. Soc., 2013, 135, 8197 CrossRef CAS PubMed; (c) A. Noole, A. Malkov and T. Kanger, Synthesis, 2013, 45, 2520 CrossRef CAS; (d) A. Awata and T. Arai, Synlett, 2013, 24, 29 CAS; (e) J. Guo, Y. B. Liu, X. Q. Li, X. H. Liu, L. L. Lin and X. M. Feng, Chem. Sci., 2016, 7, 2717 RSC.
- Examples of an asymmetric Michael-alkylation reaction in three membered ring formation: (a) G. J. Sun, J. Gong and Q. Kang, J. Org. Chem., 2017, 82, 796 CrossRef CAS PubMed; (b) B. L. Zhao and D. M. Du, Eur. J. Org. Chem., 2015, 5350 CrossRef CAS; (c) C. Kim and S. G. Kim, Tetrahedron: Asymmetry, 2014, 25, 1376 CrossRef CAS; (d) J. I. Martinez, E. Reyes, U. Uria, L. Carrillo and J. L. Vicario, ChemCatChem, 2013, 5, 2240 CrossRef CAS; (e) L. Q. Lu, J. R. Chen and W. J. Xiao, Acc. Chem. Res., 2012, 45, 1278 CrossRef CAS PubMed; (f) X. W. Dou and Y. X. Lu, Chem.–Eur. J., 2012, 18, 8315 CrossRef CAS PubMed; (g) F. Pesciaioli, P. Righi, A. Mazzanti, G. Bartoli and G. Bencivenni, Chem.–Eur. J., 2011, 17, 2842 CrossRef CAS PubMed; (h) H. Xie, L. Zu, H. Li, J. Wang and W. Wang, J. Am. Chem. Soc., 2007, 129, 10886 CrossRef CAS PubMed.
- (a) A. Noole, M. Ošeka, T. Pehk, M. Öeren, I. Järving, M. R. J. Elsegood, A. V. Malkov, M. Lopp and T. Kanger, Adv. Synth. Catal., 2013, 355, 829 CrossRef CAS; (b) R. C. da Silva, I. Chatterjee, E. Escudero-Adán, M. W. Paixăo and P. Melchiorre, Asian J. Org. Chem., 2014, 3, 466 CrossRef.
- (a) M. Ošeka, A. Noole, S. Žari, M. Öeren, I. Järving, M. Lopp and T. Kanger, Eur. J. Org. Chem., 2014, 3599 CrossRef; (b) X. W. Dou, W. J. Yao, B. Zhou and Y. X. Lu, Chem. Commun., 2013, 49, 9224 RSC.
- (a) J. H. Li, T. F. Feng and D. M. Du, J. Org. Chem., 2015, 80, 11369 CrossRef CAS PubMed; (b) A. Noole, I. Järving, F. Werner, M. Lopp, A. Malkov and T. Kanger, Org. Lett., 2012, 14, 4922 CrossRef CAS PubMed.
- J. H. Fletcher, Nomenclature of Organic Compounds, Advances in Chemistry, American Chemical Society, Washington, DC, 1974 Search PubMed.
- Selected examples of N,N′-dioxide in a catalytic asymmetric reaction, see: (a) X. H. Liu, L. L. Lin and X. M. Feng, Acc. Chem. Res., 2011, 44, 574 CrossRef CAS PubMed; (b) X. H. Liu, L. L. Lin and X. M. Feng, Org. Chem. Front., 2014, 1, 298 RSC; (c) X. H. Liu, H. F. Zheng, Y. Xia, L. L. Lin and X. M. Feng, Acc. Chem. Res., 2017, 50, 2621 CrossRef CAS PubMed; (d) K. Zheng, L. L. Lin and X. M. Feng, Acta Chim. Sin., 2012, 70, 1785 CrossRef CAS; (e) X. H. Zhao, X. H. Liu, H. J. Mei, J. Guo, L. L. Lin and X. M. Feng, Angew. Chem., Int. Ed., 2015, 54, 4032 CrossRef CAS PubMed; (f) X. Hao, L. Lin, F. Tan, C. Yin, X. Liu and X. Feng, ACS Catal., 2015, 5, 6052 CrossRef CAS; (g) J. H. Feng, L. L. Lin, K. R. Yu, X. H. Liu and X. M. Feng, Adv. Synth. Catal., 2015, 357, 1305 CrossRef CAS; (h) J. H. Feng, X. Yuan, W. W. Luo, L. L. Lin, X. H. Liu and X. M. Feng, Chem.–Eur. J., 2016, 22, 15650 CrossRef CAS PubMed; (i) J. F. Zheng, L. L. Lin, L. Dai, Q. Tang, X. H. Liu and X. M. Feng, Angew. Chem., Int. Ed., 2017, 56, 13107 CrossRef CAS PubMed.
- For more details, see the ESI.†.
- CCDC 1411858 (5aa) and CCDC 1412691 (5aa′) contain the supplementary crystallographic data for this paper.†.
- The ORTEP diagram of the catalyst had been reported in our former research, CCDC 804337 [Mg(OTf)2/L-RaPr2], CCDC 704000 [Sc(OTf)3/L-PrPr2] and CCDC 882608 [Sc(OTf)3/L-RaPr2].
- CCDC 1545249 (6aa) contains the supplementary crystallographic data for this paper.†.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 1411858, 1412691 and 1545249. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7sc02757e |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2018 |