An experienced chemistry teacher's practical knowledge of teaching with practical work: the PCK perspective
Bing
Wei
 *a and
Hao
Liu
b
*a and
Hao
Liu
b
aFaculty of Education, University of Macau, Macao. E-mail: bingwei@umac.mo
bBeijing Normal University-Hong-Kong Baptist University United International College, China
First published on 17th January 2018
Abstract
We have examined an experienced chemistry teacher's pedagogical content knowledge (PCK) of teaching with practical work in China. Based on the well-known PCK model by Magnusson S. J., Krajcik J. and Borko H., (1999), Nature, sources, and development of pedagogical content knowledge for science teaching, in Gess-Newsome J. and Lederman N. G. (ed.), Examining pedagogical content knowledge: the construct and its implications for science education, Boston: Kluwer, pp. 95–132, we focused on how the participant's teaching orientations and relevant contextual factors shaped his practical knowledge of teaching with practical work. Data from multiple sources were collected and analysed over one semester (four months), including interviews, direct classroom observation, textbooks and lesson plans. Three conclusions were drawn from this study: (1) the participant held multidimensional and mixed science teaching orientations, (2) the participant's science teaching orientations shaped his knowledge and beliefs about students’ learning and the instructional strategies related to practical work, and (3) contextual factors exerted great influence on his PCK.
Introduction
The term ‘practical work’, also called ‘laboratory work’ in North America, is used to refer to various hands-on activities or investigations involving scientific equipment or apparatus at primary and secondary schools. In recent decades, this term is often intertwined with inquiry or discovery with science curriculum development and used as an umbrella to cover various kinds of activities in science classes. In this sense, practical work can be classified into four types: confirmatory, inquiry, discovery, and problem-based (Hofstein, 2015). Due to its close association with experimentation, practical work is usually recognised as an essential part of school science teaching and learning (Hofstein and Lunetta, 2004; Hofstein et al., 2013; Wellington and Ireson, 2012). Moreover, as argued by Donnelly (1998), science teachers see the use of practical work as an essential part of what it means to be ‘a science teacher’. As a special teaching approach and learning environment, practical work has been used with a variety of expectations, often called goals or objectives, that are easily retrieved from science curriculum documents and relevant academic literature. In their comprehensive review, for instance, Hofstein and Lunetta (2004) summarised these expectations into five categories: (1) understanding of scientific concepts; (2) interest and motivation; (3) scientific practical skills and problem-solving abilities; (4) scientific habits of mind; (5) understanding of the nature of science. Very often, they continued to point out, ‘the goals articulated for learning in the laboratory have been almost synonymous with those articulated for learning science more generally’ (p. 38). It is commonly assumed that the goals and objectives of practical work and the extent to which they are achieved in the laboratory depend on a wide range of factors, such as teachers’ expectations, subject knowledge, pedagogical content knowledge (PCK), students’ abilities and interests, methods of teaching practical work (confirmatory/investigatory) and logistical and economic elements related to the availability of equipment and facilities (Hofstein et al., 2013). Some of these factors have been examined in the literature (e.g., Abrahams and Millar, 2009; Abrahams and Saglam, 2010; Nivalainen et al., 2010) whilst others have not yet been explored. In order to uncover how practical work is actually enacted in practice, we focused on practising science teachers’ knowledge, beliefs, and use of practical work in their classrooms from the perspective of PCK.PCK has held an important position since it was introduced by Shulman (1986, 1987) as a fundamental component of the knowledge base for teaching. During the past three decades, numerous researchers have elaborated on this term and described it in different ways by incorporating various components and attributes. As stated in the literature (van Driel et al., 1998), however, novice and less experienced teachers appear to lack PCK compared with their more experienced counterparts. It is thus reasonable to suggest that experienced teachers can provide more and deeper insights into the nature of the practical knowledge they possess and utilise in their teaching. Based on this consideration, this study aims to understand and illuminate what experienced chemistry teachers’ practical knowledge in the laboratory looks like. Specifically, the present single case study is guided by this question: what is an experienced chemistry teacher's PCK of teaching with practical work in a school context?
Literature review
Domain/topic-specific meanings of PCK
Although there is no universally accepted conceptualisation of PCK, agreement has been reached on two essential elements of Shulman's (1986) model: knowledge of the representations of specific subject matter and understanding of students’ learning difficulties and wrong conceptions (van Driel et al., 1998). The meaning of the term ‘subject matter’ is somewhat elusive, however, and some insights can be obtained from the discussion of subject matter knowledge in the literature. Grossman et al. (1989) defined subject matter knowledge as comprising four broad categories: (1) content knowledge – the ‘stuff’ of a discipline; (2) substantive knowledge – knowledge of the explanatory framework or paradigms of a discipline; (3) syntactic knowledge – knowledge of the ways in which new knowledge is generated in a discipline; and (4) beliefs about the subject matter – feelings and orientations towards the subject matter. On close inspection, we can see that practical work, or experimentation, is an integral part of natural sciences’ subject matter across the four categories. That is to say, practical work is a teaching approach and a kind of science subject matter as well. Moreover, according to De Jong et al. (2002), PCK can be designated at three levels: general PCK, domain-specific PCK and topic-specific PCK. As for practical work, it has both domain- and topic-specific characteristics. On the one hand, school practical work is a unique domain that is composed of particular content, scope and principles, which is quite different from subject content. On the other hand, practical work is also topic specific in that it can become nothing without specific laboratory activities. We thus decided to confer PCK with both domain and topic-specific meanings to refer to science teachers’ practical knowledge of teaching with practical work. It should be noted that while practical work is an inherent feature of school science, it is used in a variety of ways. In a general sense, practical work is often referred to as ‘prac’ or ‘pracs’ in science teaching (Wallace, 2015). For its specific forms, practical work includes teacher demonstrations and experiments conducted by the students cooperatively or individually (Hofstein, 2015). In this study, we adopt these meanings of practical work.Conceptualising PCK in teaching with practical work
PCK covers a teacher's understanding of the mechanisms for transforming disciplinary content into manifestations that are comprehensible and accessible to students (Shulman, 1987). Or, in other words, PCK is a teacher's understanding of how to help students understand specific subject matter including ‘knowledge of how specific subject topics, problems and issues can be organised, represented, and adapted to the diverse interests and abilities of learners, and then presented for instruction’ (Magnusson et al., 1999, p. 96). The concept of PCK has been interpreted, explicated, and revised by numerous science education researchers (Kind, 2009). Based on the work of Grossman (1990) and Tamir (1988), Magnusson et al. (1999) put forward a conceptualisation of PCK for science teaching that consisted of five categories: (a) orientations towards teaching science, (b) knowledge and beliefs about students’ understanding of specific science topics, (c) knowledge and beliefs about the science curriculum, (d) knowledge and beliefs about instructional strategies for teaching science, and (e) knowledge and beliefs about assessment in science. This model provides specific categories of practical knowledge for the teaching of subject matter and thus makes the concept of PCK amenable to empirical investigation. When applying this model, categories (b) through (e) specifically refer to the general domain and/or specific topic of practical work, they are ‘knowledge and beliefs about practical work in the curriculum’, ‘knowledge and beliefs about students’ learning of practical work’, ‘knowledge and beliefs about instructional strategies for teaching with practical work’ and ‘knowledge and beliefs about the assessment in practical work’.Orientations towards science teaching
Magnusson et al. (1999) contended that a teacher's orientation towards science teaching influenced all other components of a teacher's PCK. Obviously, this contention follows the line of thought that teachers’ beliefs about teaching and learning of science influence their teaching practices (Brickhouse, 1989; Laplante, 1997; Mansour, 2013). Based on an extensive review of the relevant literature, Friedrichsen et al. (2011) provided critical opinions on ‘orientation towards science teaching’ in the Magnusson et al. (1999) model. First, they pointed out that the nine different science teaching orientations identified by Magnusson et al. (1999) have mainly emanated from different curriculum traditions throughout history and lack empirical support. While commenting that this term has been vaguely defined, they argued that the link between an individual teacher's orientation towards science teaching and his/her practice had not been clearly established, arguing that the ‘relation of orientations with the other PCK components remains unclear and/or is not empirically investigated’ (Friedrichsen et al., 2011, p. 367). In many studies, they added, researchers tended to assign science teachers to one of the nine orientations described by Magnusson et al. (1999); although notable exceptions to this include the work by Grossman (1990), Volkmann, Abell and Zgagacz (2005) and Volkmann and Zgagacz (2004), who applied multiple labels to identify the science teaching orientations held by a single individual. In this study, we take the multidimensional definition of orientation towards science teaching provided by Friedrichsen et al. (2011); this consists of interrelated sets of beliefs that teachers hold in regard to the goals or purposes of teaching science, the nature of science, and science learning. Moreover, we refrain from labelling our case-study teacher simplistically as holding just one of the nine science teaching orientations noted above. Instead, the participant's orientation towards science teaching is grounded in the empirical data collected herein.PCK in context
In recognising PCK as an important category of teacher knowledge, it is important to recall the original intention of PCK. According to Shulman (1986), the teacher's knowledge base is composed of three elements: (1) content knowledge, (2) pedagogical content knowledge (PCK), and (3) curricular knowledge. In his 1987 paper, Shulman refined his three categories into a more comprehensive list (Shulman, 1987, p. 8). The crucial role of social and educational context in the formation of PCK was emphasised by Grossman (1990), who proposed a model of teacher knowledge composed of four elements: (1) subject matter knowledge, (2) general pedagogical knowledge, (3) knowledge of context, and (4) PCK. Moreover, Grossman (1990) placed PCK in the centre of the model, and represented it as influenced by and influencing the other three domains.When introducing their conceptualisation of PCK, Magnusson et al. (1999) defined PCK in the relationship between the domains of teacher knowledge suggested by Grossman (1990) in a figure and emphasised that this figure ‘is intended to depict that pedagogical content knowledge is the result of a transformation of knowledge of subject matter, pedagogy, and context’ (p. 96). Many years later, Shulman (2015) acknowledged that insufficient attention had been given to the broader social and cultural context in his original conception of PCK. As to the importance of the cultural and contextual influences of PCK, he contended that ‘culture and context are huge envelopes within which we find many of the determinants of teaching and learning’ (p. 10). He continued to emphasise that ‘PCK must be pedagogical content knowledge, but also pedagogical cultural knowledge and pedagogical context knowledge’ (p. 10). However, when examining PCK, researchers have tended to ignore the wider context within which PCK is formed, manifested and utilised, choosing to separate their investigation from that context tacitly or explicitly. In this study, we not only examine the participant's PCK of teaching with practical work, but also investigate the relationship between PCK and its context.
Based on the literature discussed above, the guiding question is refined as three research questions:
(1) What are the participant's teaching orientations when teaching with practical work?
(2) What is the participant's practical knowledge of teaching with practical work?
(3) In what ways do the participant's teaching orientations influence his practical knowledge of teaching with practical work in context?
Methodology
This explorative investigation takes place within a qualitative case study research paradigm, which is ‘an intensive, holistic description and analysis of a single instance, phenomenon, or social unit’ (Merriam, 1998, p. 21). We focus on a single case study, which allows us to explore in detail the phenomena, i.e., an experienced chemistry teacher's practical knowledge of teaching with practical work, through exhaustive descriptions and in-depth data collection from multiple sources of information in a school context.Participant and context
The participant in this study, John (alias), was an experienced chemistry teacher with approximately 30 years of work experience; he was a chemical laboratory technician for four years and had been a chemistry teacher for 26 years. This was the main reason why he was purposively selected as the participant in this study. In addition to his rich experience in teaching, John was well regarded in his school, and his teaching was frequently referred to as adhering to good practice standards. Therefore, he could be considered as a typical case in terms of both representativeness and informativeness (Yin, 2009). John graduated from a specialist teacher training university in mainland China and holds a bachelor's degree in science. The school John worked in was recognised as one of the best among senior high schools (Grades 10, 11 and 12) in a coastal city in southern China by virtue of its high enrolment rate in the College Entrance Examinations (CEE), or Gaokao. John was the head of the chemistry department at this school and he taught Grade 10 students; there were 1300 students at this grade distributed across 26 classes with approximately 50 students per class. There were nine chemistry teachers, including John, plus four lab technicians. Generally, each teacher was responsible for three classes in this senior high school. At the time this study was conducted, John taught three Grade 10 classes with a total of nine class sessions per week, each lasting 40 minutes. The chemistry textbooks used in this school were compiled by Song et al. (2007), in accordance with the 2001 Chinese curriculum reform† (Wei, 2005, 2012).Data collection
To investigate the participant's practical knowledge in chemistry laboratory teaching, data from multiple sources were collected and analysed over a period of one semester (four months), including interviews, direct classroom observation, textbooks, and lesson plans. In terms of the former, two planned semi-structured interviews were carried out with John, and these constituted our primary source of data. A protocol derived from the theoretical framework of Magnusson et al. (1999) was prepared before the interviews; questions were asked based on the main points listed in that protocol. In the first formal interview, the participant was first asked to describe his experiences in life and routine work related to chemistry teaching and chemistry laboratory work in particular, and then the interview was focused on his knowledge and beliefs about the goals and purposes of chemistry teaching, the nature of chemistry/science and chemistry learning, and the features of practical work. Specifically, the interview was guided by these four questions: (1) what is your purpose in chemistry teaching? (2) What is your opinion on effective chemistry teaching? (3) What are the characteristics of chemical experiments compared with chemistry content knowledge? (4) How do you comment on the differences and commonalities between practical work and inquiry? The second formal interview was particularly focused on the four aspects of PCK. Referring to ‘content representation’ suggested by Loughran et al. (2004), we prepared four interview questions in advance: (1) What are the purposes and actual roles and functions of practical work in your teaching? (2) What is your knowledge about students’ learning difficulties and problems concerning practical work? (3) How do you teach with practical work, and (4) how do you evaluate your students’ performances in practical work? These two formal interviews were conducted at the initial stage of data collection and some points were probed further at the later stage with instances and episodes of teaching with practical work observed in John's class. In communicating with John, sometimes practical work was used as a general term and sometimes it was used to refer to specific practical activities or experiments. Before the first formal interview, the purpose of our research was introduced and explained to John, and he signed a consent form that included details of our obligations vis-à-vis his privacy.In addition to the formal interviews, pre- and post-class interviews were arranged with almost all of the class observations. Pre-class interviews were used to understand the teacher's thinking about the design and purpose of a laboratory teaching activity on a specific topic. Post-class interviews were mainly used to understand the practical knowledge gained by John through reflection on his teaching practices and experiences. Classroom observation was used to provide direct data on how practical work was carried out and the scale and scope of interactions between the teacher and his students in the class. In total, 28 class sessions were observed during the period of data collection. All of the classes we observed involved practical work, either teacher demonstrations or student experiments, with different classes of students. On average, two classes were observed per week during the period of study. The observation and the accompanied pre-post interviews constituted the second major source of data in this study.
Data analysis
The interview transcripts and observation notes were analysed using qualitative content analysis (QCA), which is a method for systematically describing the meaning of qualitative material through classifying instances into categories of a coding frame (Schreier, 2012). The coding frame consists of principal categories or dimensions specifying relevant aspects with subcategories specifying relevant meanings (Schreier, 2012). In this paper, the term ‘categories’ and ‘dimensions’ are synonymous and used interchangeably. The five aspects of the theoretical framework for PCK proposed by Magnusson et al. (1999) served as the main categories or dimensions of the heuristic coding frame. Some subcategories were also drawn from the work of Magnusson et al. (1999), such as ‘inquiry’ and ‘academic rigor’, which were two subcategories under the dimension of the orientations of science teaching. However, most of the subcategories we operationalised were derived and defined based on the data collected; for instance, ‘demonstration vs. student experiments’ and ‘investigative vs. confirmatory practical work’ under the dimension of knowledge of instructional strategies. In this study, a ‘thematic criterion’ (Schreier, 2012, p. 136) was used for data segmentation; that is, five aspects of John's PCK of teaching with practical work were demarcated. The segments/units were identified and marked according to the thematic criterion; an emergent coding system was established during and through data analysis. Due to the limited space, we only report two aspects of the PCK of practical work in this paper, they are John's knowledge and beliefs about ‘students’ learning of practical work’ and ‘instructional strategies for teaching with practical work’. The categories and subcategories of the participant's PCK of teaching with practical work are presented in Table 1.| Categories/dimensions of PCK | Subcategories |
|---|---|
| Orientation of science teaching | • Inquiry |
| • Academic rigor | |
| • Hands-on activities | |
| Students’ learning in practical work | • Understanding of chemical principles |
| • Manipulative skills | |
| • Improper attitude toward practical work | |
| Instructional strategies of practical work | • Demonstration vs. student experiments |
| • Investigative vs. confirmatory practical work |
Trustworthiness
The instrumental and content-specific quality of the coding frame developed in this study was assessed in terms of the reliability and validity considerations. To assess the reliability of the coding frame, a strategy of comparisons across points in time (Schreier, 2012) was used, by which we used the same coding frame to analyse the same units of coding across points in time until complete consistency was reached. To establish validity, first, multiple data sources allowed triangulation of both data collection and analysis, and comparisons were regularly made to confirm consistency between perspectives, actual behaviour in classes/practical sessions, instruments, and other phenomena used in the practical classes and curriculum materials. This allowed us to identify any inconsistencies between espoused ideas and actions in practice. Second, we conducted member checks and obtained participants’ confirmation vis-à-vis our analytical approaches and interpretations. For example, when we could not understand the term shiyan in Chinese (experiment/experimenting), which was often mentioned in interviews, we sent the transcripts to the participants to clarify whether it meant ‘teacher demonstration’, ‘student group work’ or ‘scientific experiment’. Third, throughout the process of data collection and analysis, the two authors coded the data independently, and any differing opinions were discussed and resolved to achieve consensus.Findings
The presentation of the findings of this study is mainly based on the categories and subcategories listed in Table 1. Specifically, John's PCK of teaching with practical work in the school context is divided into two parts as follows: (1) multidimensional and mixed science teaching orientations, and (2) practical knowledge of teaching with practical work.Multidimensional and mixed science teaching orientations
In John's opinion, the fundamental purpose of chemistry teaching was to develop students’ abilities of thinking scientifically, that is, the thinking habit of inquiring into the source of scientific knowledge. He believed that students should learn to think scientifically through the learning of chemistry and other natural sciences. If students possessed the ability of scientific thinking, he added, they would be able to identify the crux of problems and suggest appropriate solutions. As for the purpose of inquiry, he stated that it was the process of developing students’ thinking abilities. By using the inquiry learning method, students could perform well in exams, with their learning burdens reduced correspondingly. For teachers, John continued, inquiry was a way of teaching. In his opinion, teachers usually relied on two approaches to make students acquire new knowledge: delivering and guiding. Therefore, whether students made inquiries depended on the approach adopted by teachers: guiding students to discover knowledge and imitate the discovery processes of key predecessors, or just delivering knowledge to students directly. John's understanding of inquiry was exhibited in his comments on the current chemistry textbooks used in his class. In the old textbook, John pointed out, chemical formulas were generally given directly following the expression of ‘according to theory…’ while in the new textbook they were generally derived from experiments and theories. Specifically, John took the section of ‘molar volume of gas’ (Song et al., 2007, p. 13) as an example: an experiment was first carried out in line with the principle of electrolysis of water. Students then observed the volume ratio of O2 and H2 formed. Finally, the volumes of 1 mol of O2 and 1 mol of H2 in standard conditions were calculated.As for the relationship between practical work and the chemistry subject matter, John believed that much of the knowledge in chemistry was derived from experimentation, that is, many concepts and principles could be found through chemical experiments. Moreover, he believed in a ‘structure of chemistry’ that constituted the basic content of chemistry teaching. Experimentation consisted of various kinds of scientific methods. Among those methods, the design of experiments was important because scientists used it to find the means by which they wanted to achieve their goals. John continually emphasised that experimental design should be logical and rigorous, which involved not only basic manipulative skills but also chemical principles. Neither manipulative skills nor chemical principles could be ignored in the process of experimentation. Therefore, he said, helping students learn to design experiments was an important part of laboratory teaching.
As John commented, his colleagues recognised the role and importance of laboratory teaching to different extents because of their differential life experiences, their educational philosophies, and the prevailing education system. He acknowledged definitively that he had a preference for practical work in his teaching practice, and he thought that this preference was mainly rooted in his life experiences over time.
I believe I have a strong hands-on ability because in my daily life, anything that I am capable of doing myself, I will do myself. For instance, when the light doesn’t work or the drains are blocked, I always choose to solve these problems by myself. I am in favour of DIY. At the time when I was growing up, almost all of the toys were made by us. Sometimes, when we saw the handmade toys of others, we would look into the details of their structures and materials and then reproduce the toys at home. During the process of imitation, we might even think about how to make improvements, and then we compared our replicated products with those of buddies and got a sense of achievement.
Based on his own hands-on experiences, he thought that turning unknowns into knowns through one's own instincts and actions not only enhanced one's personal abilities and understanding of the subject matter, but also induced a sense of achievement. Only students who were trained in this way could be endowed with real creative capacity. John explained his idea using a domestic plumbing example: ‘Once we did it, we would understand what its structure was, why it was designed like this, and what we should do if it did not work’.
Taking the nine orientations of Magnusson et al. (1999) as a reference, we can conclude that John's orientations featured the characteristics of ‘inquiry’, ‘academic rigor’ and ‘activity-driven’ to some degree. First, he thought that the main goal of practical work was to investigate the source of knowledge, and he preferred to use the teaching method of guiding students to discover knowledge, allowing them to imitate historical predecessors in the process of discovery. Second, he believed in the ‘structure of chemistry’ and thought that many chemical principles were discovered through laboratory work, which involved not only chemical principles but also basic manipulative skills. Third, he was a proponent of hands-on activities from his own growth experiences and believed that these activities not only enhanced personal abilities but also instilled an important sense of achievement. From the data we collected, we cannot conclude that John typically and systematically held any of the nine orientations of science teaching defined by Magnusson et al. (1999). Instead, we can conclude that he embraced reform-focused teaching orientations, such as inquiry, whilst opposing traditional teaching orientations, such as a didactic method of instruction.
Practical knowledge of teaching with practical work
Students all know that sodium carbonate solution can react with calcium hydroxide or calcium chloride solution to form white precipitates. They also know the reaction between sodium bicarbonate and calcium chloride will not form precipitates, as bicarbonate radicals and calcium ions will not form insoluble substances. As to the reaction between sodium bicarbonate and calcium hydroxide solution, however, students may subjectively consider that there will be no precipitates.
As John explained, the problem in this instance was that students were not familiar with the principles of the chemical reaction. To solve this problem, John carried out an experiment in class, helping students recognise their misconceptions through their own observations and guiding them to think about what reaction occurred and judge whether the calcium hydroxide could be used to distinguish sodium carbonate from sodium bicarbonate. He commented that chemical principles served as threads that strung various kinds of specific chemical reactions together; thus, those students doing well in chemistry could fully understand the principles behind a certain group of chemical reactions instead of a single specific reaction and could therefore avoid rote memorisation. As for the current status of students’ learning in Grade 10 compared with the previous year, when they were junior three (Grade 9) students, John provided the following comments:
Chemistry in junior three only required students to understand some basic substances and their properties, which could be learned by rote memorisation. When coming to senior one, some students still tried to memorise everything but ended up with low grades. Thus, they thought chemistry in senior high school was very difficult compared with that in junior three. In fact, chemistry in senior one has become more demanding with respect to understanding of the principles.
John thought that manipulative skills were very important in conducting practical work. According to him, each apparatus required particular standardised manipulation skills. However, as he observed, many students did not know how to correctly operate in practical work:
For instance, when a chemical reaction happened in a test tube, it required the liquid to be mixed uniformly in the tube, so students needed to shake the tube by swinging their wrist, but most students did not do it properly. Some shook up and down, and some drew circles. In the case of a conical flask, you cannot shake it because it is too big and will not work that way; instead, you need to agitate the flask.
In John's opinion, the main reason for most difficulties experienced by students in chemistry practical work was the lack of enough hands-on opportunities. As he pointed out, class sizes in the school tended to be large, around 50 students per class; thus, there were no ample opportunities for each and every student to exercise basic manipulative skills.
Moreover, as John observed, another problem related to students’ attitudes towards laboratory work. Generally speaking, he said, students tended to hold improper attitudes, perceiving themselves as onlookers watching something fun as the teachers demonstrated the laboratory work. In an interview after the class on ‘several metallic compounds’, where a couple of demonstrations were conducted, John was obviously not satisfied with students’ learning state in the class and gave the following comments:
As you have seen, many students acted like they were watching the circus. They relaxed their brains to watch the show of the teacher, so they may remember the conclusion and phenomena but forget other aspects. The more exciting the laboratory work was, the more interested the student were, but they did not understand what I was doing.
John drew an analogy between watching teachers’ demonstrations and watching fireworks to indicate that, even after watching, many students may not know the purpose of doing the experiment or how to do it. John believed that students’ whimsical attitudes in this respect were a direct consequence of the current educational system, particularly the CEE, which put too much emphasis on performance on paper examinations. Because of this, he added, students who were inept at hands-on activities could nevertheless do well on exams as long as they had good memorisation skills.
Instructional strategies of practical work
Demonstration vs. students’ experiments. During the period of our class observations, John performed demonstrations in almost every class. His lesson plans were filled with experimental sketches and he was always willing to talk about his intentions and the actual effects of his demonstrations. Most of these demonstrations were based on the various activities prescribed in the textbook, but some he had developed himself, such as the water purifier he designed in the session entitled important metallic compounds. According to John, if students did not obtain perceptual knowledge through practical work, they would lack important cognitive development and skills, especially in the situation of acquiring new knowledge. Thus, making the phenomena of experiments more observable was John's major concern when conducting practical work. In the class entitled investigating metals’ reactions with water and acid solutions, the reaction between iron powder and vapour was demonstrated twice and then shown in a video. As John explained in the post-class interview, this experiment was generally associated with less obvious phenomena; he made a video of himself carrying out the experiment in advance, in case he deemed it necessary for use as a supplementary aid. Indeed, because of the phenomenological concerns previously noted, he finally played the video for students in class. Moreover, as he stated in the first formal interview, students’ experiments were more effective when they were provided with direct perceptual experiences, stimulating the senses from watching, hearing, touching, and smelling. Some abstract chemical knowledge, such as the difference between solution and gel, he continued, was hard for students to understand and memorise without the act of doing. Therefore, John said the ideal condition for laboratory teaching would be for students to carry out all practical work prescribed in the curriculum. However, class observations showed that students received far fewer opportunities to do practical work than to watch demonstrations or videos. This is consistent with what he said in the second formal interview, when asked whether he preferred the demonstration experiments to the student experiments when teaching with practical work, John answered in the affirmative. In addition to health and safety considerations, John explained that time constraints were important to consider:Yes, it saves time. The course schedule is very tight. Meanwhile, many class hours have been spent on other things such as holidays, exams, and sports days. Take my lecture as an example. One lesson has three lectures a week, but sometimes only one lecture can be offered due to the National Day or the Mid-Autumn Festival; moreover, the diagnostic test takes one week. It is really hard for us to do something else in a subject like chemistry without sufficient time.
Our field study discerned that aside from regular class hours, there was a dedicated class for doing exercises or explaining homework problems and mock exam questions by teachers to consolidate the knowledge learned. In addition, aside from the diagnostic tests mentioned above, quizzes were arranged following the completion of a particular chapter or knowledge point. Therefore, exercises and exams took up a lot of class hours. As we observed, student experiments were carried out in the classroom directly along with regular teaching, instead of in the laboratory, after the teaching had been finished in class. Experiments were moved from the laboratory to the classroom mainly because a lot of time could be saved from shuttling between classroom and laboratory. As to the arrangement of laboratory work, working groups were formed conveniently by students sitting adjacent at front and back desks, and an experimental apparatus was distributed to each group in advance before the class, which could also save on time.
Investigative vs. confirmatory practical work. For practical work, in John's view, the process was more important than the result; ‘if the process is understood, the ultimate conclusion can be generated naturally with the procedural knowledge obtained’. In John's mind, in a sense, each chemistry class was an inquiry process that investigated the properties of unknown substances and demonstrated them to students through practical work. When investigating a problem or a dilemma, John first asked students to think about how to design an experiment to reach a conclusion by brainstorming. As planned, there was a section for ‘scientific inquiry’ in the Investigating metals’ reactions with water and acid solution class. We learned from the pre-class interview that John planned to ask students to design the apparatus required for the reaction between iron powder and vapour. In this case, John gave hand-outs to students in advance for their preparation. The main task was device design, and this was a pre-lab assignment. He asked students to finish the task one day in advance, and then took photos of their designs and uploaded them to a computer for display in class. Moreover, John made some improvements on the activities prescribed in the textbook. In the textbook (Song et al., 2007, p. 50), there were only two experiments on metals’ reactions with acid and water: the reaction between sodium (Na) and water and the reaction between iron powder (Fe) and vapour. Reactions between potassium (K), magnesium (Mg), and aluminium (Al) with water were not covered. In line with the Metal Activity Sequence Table, John selected some representative elements by metal activity, including K, Na, Mg and Al, to investigate the general characteristics of their reactions with water and acid. After class, John reflected that it was a very typical chemistry class, with a typical process summarising principles and grasping the essence through watching and analysing in practical work.
According to John, however, not all chemical knowledge was suitable for using the inquiry approach; some knowledge must be taught by teachers. In the session entitled oxides of sulphur and oxygen, a ‘scientific inquiry’ activity is set in the textbook to ask students to conduct an experiment of absorbing as much nitrogen dioxide into water as possible (Song et al., 2007, p. 92). However, this investigation was not actually adopted in John's class. When asked for the reasons, John gave the following explanations:
First, as you know, nitrogen dioxide was not easily prepared in the laboratory. That is to say, we could not provide students with nitrogen dioxide as suggested in the textbook. Second, the intention of this experiment is to let students know that nitrogen dioxide dissolve in water very easily. I can tell them this conclusion directly. Actually, this conclusion is presented in the textbook clearly. It's enough.
More importantly, as he indicated later, this inquiry needs a lot of time. To ensure teaching is on schedule, students can only be offered a few opportunities to make real inquires. John pointed out that inquiry-oriented experiments could only be viable if time was not a constraint as in normal classes. Indeed, as we counted, the number of investigatory experiments was only 5 during the period of our field study. In John's view, practical work as a supplementary teaching approach could help students construct a knowledge system with the role of testing and proving, a process of confirmation of the knowledge learned in class rather than inquiry. He explained this point further as follows:
Verification and inquiry supplement each other and are both necessary. Facing the college entrance exam (CEE), we cannot require students to investigate everything. Inquiry is good for developing scientific thinking, but a complete knowledge system cannot be constructed only on the basis of practical work and inquiry, as the current mode of college entrance exam requires students to construct a complete knowledge system and laboratory work is only a supplementary approach in many cases.
Discussion
In this study, we used a case study approach to investigate an experienced chemistry teacher's practical knowledge of teaching with practical work from the PCK perspective. In the previous section, John's orientations of chemistry teaching and his practical knowledge of students’ learning difficulties and instructional strategies related to practical work were reported. Based on these findings, three issues are discussed in this section: possible sources of John's orientations of chemistry teaching; the ‘shaping’ roles played by teaching orientations on John's knowledge and beliefs about students’ learning problems and instructional strategies related to practical work; and the contextual factors that exerted influences on John's PCK related to practical work.First, we suggest that John's multidimensional and mixed orientations of chemistry teaching emanated from different sources. The first was the reform of the science curriculum. In the last two decades, science curriculum innovations and reforms have been initiated by the central government in China, accompanied by new instructional ideas and student-centered pedagogy imported from the West (Wei, 2010). To effectively implement the new science curricula, a variety of in-service science teaching training programmes and projects have been provided at the national, provincial, and local levels (Ding, 2015). John might have developed his reform-oriented teaching ideas and pedagogy through this channel. The second possible source was John's own perceptions of the nature of chemistry, chemistry teaching, and chemistry learning. For example, he believed that the subject of chemistry possessed a ‘structure’ underpinned by basic concepts and principles; if students mastered these concepts and principles, they would find it easier to learn chemistry. John's early experiences constituted the third source of his chemistry teaching orientation; this was manifested in his advocating hands-on activities in chemistry teaching. It was his early experiences with inspecting and re-creating handmade toys as a boy that made him recognise the significance of hands-on activities in the process of student learning.
Consistent with the results of other researchers who have applied the Magnusson et al. (1999) model (e.g., Demirdöğen and Uzuntiryaki-Kondakci, 2016), evidence has supported the claim that a teacher's orientation towards science teaching has shaped the genesis and content of four aspects of PCK. In this study, we detected the ‘shaping’ roles played by John's teaching orientations on his knowledge and beliefs about students’ learning problems and difficulties and instructional strategies related to practical work. First, John's practical knowledge about students’ learning in practical work was mainly influenced by his ‘academic rigor’ orientation of science teaching. As demonstrated earlier, John's knowledge and beliefs about students’ learning problems in practical work were related to the students’ understanding of chemical principles, manipulative skills, and improper attitudes. As we have also known, John put much emphasis on the ‘structure’ of chemistry, believing that ‘if students mastered the principles, they could even get the key results without doing experiments’ while he highly valued manipulative skills. Correspondingly, the former point can be used to explain his knowledge and beliefs of students’ learning of chemical principles, and the latter is somewhat associated with his knowledge and beliefs of students’ weaknesses and limitations in terms of operating and using chemical equipment and apparatus. Second, John's ‘hands-on’ teaching orientation influenced his instructional strategies in practical work. The empirical data of this study showed that John was keen on providing students with access to practical work, through either teacher demonstrations or the students’ own experiments. In terms of demonstrations, John spared no effort to make the phenomena more observable. For students’ experiments, his intention was to develop their perceptual knowledge. Third, John's orientations of inquiry and ‘academic rigor’ were reflected in his treatment of investigative and confirmatory practical work. Specifically, in his classes, John was often observed to guide students to find answers and solve problems through investigative experiments, but he also believed that ‘not all chemical knowledge was suitable for employing the inquiry approach’ and ‘some knowledge must be taught by teachers’.
Some, if not all, of John's knowledge and beliefs about the two aspects of his PCK of teaching with practical work were determined by contextual factors. The nature of these associations was dependent on the nature of the interactions between certain aspects of his PCK and those contextual factors. While acknowledging that John's science orientations were to some degree related to context, the following discussion is focused on the two aspects of his PCK. First, as we know, practical work, in the form of either domain- or topic-specific subject matter, has never existed separately but is always part of the existing curriculum. As our observation showed, John's selection and arrangement of practical work was always anchored in the existing curriculum and textbooks. As for students’ learning of practical work, as John observed, students were often whimsical about practical work, which was a direct consequence of the exam-oriented education system, specifically the CEE. The large class sizes comprised another reason. As John explained, due to the large class sizes, not all students had sufficient opportunities to practise and develop their manipulative skills. Moreover, time constraints were the primary contextual factor that influenced John's knowledge and beliefs about instructional strategies. As shown, John was keen on conducting practical work in his teaching and stated that in an ideal situation the students would conduct all practical work. In reality, however, his demonstrations occurred more frequently than students’ experiments due to time constraints. This contextual factor also constrained him from organising inquiry-oriented practical work; that is, ‘to ensure teaching on schedule’, the number of investigatory experiments had to be curtailed. In summary, the contextual factors include the existing curriculum and textbooks, CEE, large class sizes, and time constraints.
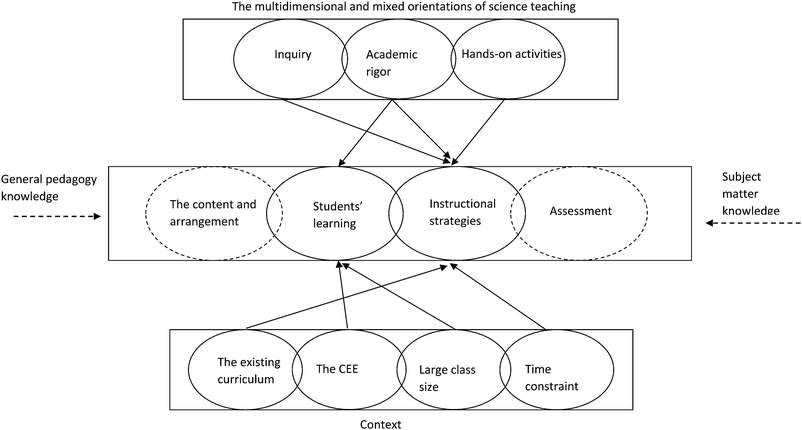
Based on the foregoing discussion, we can visualise relationships between the three issues that are involved in John's practical knowledge of teaching with practical work in Fig. 1 (see below). In Fig. 1, from top to bottom, the three boxes represent John's orientations of science teaching, practical knowledge of teaching with practical work, and context, respectively. The ellipses represent John's individual science teaching orientations, the four aspects of PCK, and the contextual factors, while the overlaps between the ellipses denote the intersections between them. Since the ‘curriculum’ and ‘assessment’ are not the focus of this paper, we use two dashed ellipses to represent them. The arrows at the end of the solid lines indicate the influencing roles exerted by teaching orientations and contexts. Of course, we would not like to exclude the influencing roles played by general pedagogical knowledge and subject matter knowledge; these were identified in previous studies (Grossman, 1990; Gess-Newsome, 1999; Magnusson et al., 1999) and are beyond the scope of this study. Thus, the arrow at the ends of the two broken lines are used to represent such influences.
From this model, some references can be made for other individual science teachers. First, for the domain/topic of practical work, an individual science teacher's orientations can be multiple and mixed, ranging from reforming (such as scientific inquiry) to traditional (such as academic rigor). Second, a science teacher's orientations shape his/her practical knowledge and beliefs about students’ learning and instructional strategies related to practical work. However, the shaping mechanism is complicated by different orientations ‘shaping’ different aspects of practical knowledge (see Fig. 1). In this study, for example, John's inquiry orientation shaped his knowledge and beliefs about instructional strategies, while his ‘academic rigor’ shaped his knowledge and beliefs about students’ learning and instructional strategies. Third, the shaping roles are mediated by contextual factors, which include the official curriculum and textbooks, large class sizes, time constraints, and the CEE. Unfortunately, as observed in this study, most of these contextual factors exerted negative influences on the effective enactment of practical work.
This study provides a concrete example of an experienced chemistry teacher's PCK of teaching with practical work. Rarely is practical work treated as part of the subject matter of chemistry and few previous research studies have addressed this topic in the view of PCK. In this sense, this study contributes to the research base of PCK in the field of chemistry education and even in the field of science education by extending the view of subject matter knowledge. However, we must say that PCK is an idiosyncratic feature of a science teacher and it is complicated, dynamic, and flexible (Kind, 2009; van Driel, 2015). It is thus not realistic to generalize this conclusion to other chemistry teachers in other countries. Despite all this, the findings of and insights into John's PCK of teaching with practical work in China could be used as inputs in the development of science teachers’ PCK of teaching with practical work. First, as shown in this study, an individual chemistry teacher's orientations of teaching are multidimensional and mixed, including both contemporary and conventional views of science teaching, and different orientations of science teaching shaped different aspects of PCK. This result should be reflected in pre-service and in-service science education programmes to inform science teachers of the neutral nature of practical work in that it can be used to promote both inquiry-oriented and traditional science teaching. Preferably, science teachers should be provided with opportunities to learn how to transform traditional practical work into scientific experiments with a reasonably conceptual, epistemological, and procedural foundations in the view of science practice (NRC, 2012; Erduran and Dagher, 2014; Stroupe, 2015). Second, this study has demonstrated that a chemistry teacher's PCK is context specific, and sometimes constrained by various contextual factors in the process of developing PCK of teaching with practical work. Hence, we suggest that social communities and school administrations should use their resources and influences to help foster facilitative work environments in which science teachers have opportunities to develop reform-minded identities (Luehmann, 2007), with positive attitudes towards and active participation in inquiry-based practical work being encouraged and supported.
Conflicts of interest
There are no conflicts to declare.References
- Abrahams I. and Millar R., (2009), Practical work: making it more effective, Sch. Sci. Rev., 91(334), 59–64.
- Abrahams I. and Saglam M., (2010), A study of teachers’ views on practical work in secondary schools in England and Wales, Int. J. Sci. Educ., 32(6), 753–768.
- Brickhouse N. W., (1989), The teaching of the philosophy of science in secondary classrooms: case studies of teachers’ personal theories, Int. J. Sci. Educ., 11, 437–449.
- De Jong O., Veal W. R. and Van Driel J. H., (2002), Exploring chemistry teachers’ knowledge base, in Gilbert J. K., De Jong O., Justi R., Treagust D. F. and Van Driel J. H. (ed.), Chemical education: towards research-based practice, The Netherlands: Kluwer Academic Publishers, pp. 369–390.
- Demirdöğen B. and Uzuntiryaki-Kondakci E., (2016), Closing the gap between beliefs and practice: change of pre-service chemistry teachers’ orientations during a PCK-based NOS course, Chem. Educ. Res. Pract., 17, 818–841.
- Ding B., (2015), Science Teacher Education in Mainland China, in Gunstone R. (ed.), Encyclopedia of Science Education, New York: Springer, pp. 917–924.
- Donnelly J., (1998), The place of the laboratory in secondary science teaching, Int. J. Sci. Educ., 20(5), 585–596.
- Erduran S. and Dagher Z., (2014), Reconceptualizing the nature of science for science education, New York: Springer.
- Friedrichsen P., van Driel J. H. and Abell S. K., (2011), Taking a closer look at science teaching orientations, Sci. Educ., 95(2), 358–376.
- Gess-Newsome J., (1999), Pedagogical content knowledge: an introduction and orientation, in Gess-Newsome J. and Lederman N. G. (ed.), Examining pedagogical content knowledge: the construct and its implications for science education, Boston: Kluwer, pp. 3–20.
- Grossman P. L., (1990), The making of a teacher: teacher knowledge and teacher education, New York, NY: Teachers College Press.
- Grossman P. L., Wilson S. M. and Shulman L. S., (1989), Teachers of substance: subject matter knowledge for teaching, in Reynolds M. C. (ed.), Knowledge Base for the Beginning Teacher, New York: Pergamon, pp. 23–36.
- Hofstein A., (2015), Forms of laboratory work, in Gunstone R. (ed.), Encyclopedia of Science Education, New York: Springer, pp. 563–564.
- Hofstein A. and Lunetta V. N., (2004), The laboratory in science education: Foundations for the twenty-first century, Sci. Educ., 88, 28–54.
- Hofstein A., Kipnis M. and Abrahams I. Z., (2013), How to learn in and from the chemistry laboratory, in Hofstein A. and Eilks I. (ed.), Teaching Chemistry – A Studybook, Netherlands: SENSE, pp. 153–182.
- Kind V., (2009), Pedagogical content knowledge in science education: perspectives and potential for progress, Stud. Sci. Educ., 45(2), 169–204.
- Laplante B., (1997), Teachers’ beliefs and instructional strategies in science: pushing analysis further, Sci. Educ., 81, 277–294.
- Loughran J.J., Mulhall P. and Berry A., (2004), In search of pedagogical content knowledge in science: developing ways of articulating and documenting professional practice, J. Res. Sci. Teach., 41, 370–391.
- Luehmann A., (2007), Identity development as a lens to science teacher preparation, Sci. Educ., 91, 822–839.
- Magnusson S. J., Krajcik J. and Borko H., (1999), Nature, sources, and development of pedagogical content knowledge for science teaching, in Gess-Newsome J. and Lederman N. G. (ed.), Examining pedagogical content knowledge: the construct and its implications for science education, Boston: Kluwer, pp. 95–132.
- Mansour N., (2013), Consistencies and inconsistencies between science teachers’ beliefs and practices, Int. J. Sci. Educ., 35(7), 1230–1275.
- Merriam S. B., (1998), Case study research in education: a qualitative approach, San Francisco, CA: Jossey-Bass.
- National Research Council (NRC), (2012), A science framework for K-12 science education: practices, crosscutting concepts, and core ideas, Washington, DC: The National Academies Press.
- Nivalainen V., Asikainen M., Sormunen K. and Hirvonen P. E., (2010), Preservice and inservice teachers’ challenges in the planning of practical work in physics, J. Sci. Teach. Educ., 21, 393–409.
- Schreier M., (2012), Qualitative content analysis in practice, London: SAGE Publications Ltd.
- Shulman L. S., (1986), Those who understand: knowledge growth in teaching, Educ. Res., 15(2), 4–14.
- Shulman L. S., (1987), Knowledge and teaching: foundations of the new reform, Harvard Educ. Rev., 57(1), 1–22.
- Shulman L. S., (2015), PCK: Its genesis and exodus, in Berry A., Friedrichsen P. and Loughran J. (ed.), Re-examining pedagogical content knowledge in science education, New York: Routledge, pp. 1–13.
- Song X. Q., Wang J., Zheng C. L., Li W. D. and Wang Z. M., (2007), Chemistry I, 3rd edn, Beijing: People's Education Press.
- Stroupe D., (2015), Describing ‘science practice’ in learning settings, Sci. Educ., 99, 1033–1040.
- Tamir P., (1988), Subject matter and related pedagogical knowledge in teacher education, Teach. Teach. Educ., 4, 99–110.
- van Driel J. H., (2015), Pedagogical content knowledge in teacher education, in Gunstone R. (ed.), Encyclopedia of Science Education, New York: Springer, pp. 736–737.
- van Driel J. H., Verloop N. and De Vos W., (1998), Developing science teachers’ pedagogical content knowledge, J. Res. Sci. Teach., 33(6), 673–695.
- Volkmann M. and Zgagacz M., (2004), Learning to teach physics through inquiry: the lived experiences of a graduate teaching assistant, J. Res. Sci. Teach., 41, 584–602.
- Volkmann M., Abell S. and Zgagacz M., (2005), The challenges of teaching physics to preservice elementaryteachers: orientations of the professor, teaching assistant, and students, Sci. Educ., 89, 847–869.
- Wallace J., (2015), Practical work, in Gunstone R. (ed.), Encyclopedia of Science Education, New York: Springer, p. 763.
- Wei B., (2005), Science curriculum reform in post-compulsory education in the People's Republic of China: the case of senior secondary school chemistry curriculum, Sci. Educ. Int., 16, 291–303.
- Wei B., (2010), The changes in science curricula in China after 1976: a reflective review, in Lee J. (ed.), Science Education Research in Asia, Boston: Sense Publishers, pp. 89–102.
- Wei B., (2012), Chemistry curriculum reform in China: policy and practice, in Yin H. and Lee C. J. (ed.), Curriculum reform in China: Changes and challenges, New York: Nova Science Publishers, Inc., pp. 95–109.
- Wellington J. and Ireson G., (2012), Science learning, science teaching, 3rd edn, London: Routledge.
- Yin R. K., (2009), Case study research: design and methods, 4th edn, Thousand Oaks, CA: Sage Publications.
Footnote |
| † The chemistry curriculum at senior high schools is divided into compulsory and elective elements. Correspondingly, there are both compulsory and elective chemistry textbooks. The tenth graders taught by John were using compulsory textbooks during the period of this study. Subsequently, in their first semester as eleventh graders, they would be divided into liberal arts and science tracks. Only students following the latter track would continue to study the elective chemistry curriculum and then proceed to take the CEE chemistry exam. |
| This journal is © The Royal Society of Chemistry 2018 |