Teaching assistants' topic-specific pedagogical content knowledge in 1H NMR spectroscopy
M. C.
Connor
and
G. V.
Shultz
 *
*
Department of Chemistry, University of Michigan, 930 N. University, Ann Arbor, MI 48109, USA. E-mail: gshultz@umich.edu
First published on 3rd April 2018
Abstract
Nuclear magnetic resonance (NMR) spectroscopy is an essential analytical tool in chemistry, and the technique is routinely included as a topic across the undergraduate chemistry curriculum. As a result of NMR's importance, classroom instruction of this topic has received considerable attention in chemistry education research. However, little is known about instructors’ knowledge for teaching this topic. In order to better understand this knowledge, we investigated topic-specific pedagogical content knowledge in 1H NMR spectroscopy among 20 chemistry teaching assistants at a large Midwestern university in the United States. A questionnaire was developed to provide an inferential measure of content knowledge and topic-specific pedagogical content knowledge in 1H NMR spectroscopy for participants with a range of teaching experience. Data from the questionnaire were analyzed qualitatively and quantized using a rubric. The quantitative data were transformed using the Rasch model and statistically analyzed. Results from these analyses indicate that pedagogical content knowledge increased with teaching experience in 1H NMR spectroscopy, suggesting that knowledge for teaching this topic is developed through practice. Additionally, the development of pedagogical content knowledge was found to depend upon content knowledge required for specific NMR sub-topics and problems. This finding suggests that the ultimate “grain-size,” or domain-specificity, of pedagogical content knowledge may extend to the problem level. Results from this study have implications for how instructors may cultivate knowledge for teaching NMR spectroscopy, as well as for how pedagogical content knowledge may be more effectively incorporated into instructor training programs.
Introduction
Nuclear magnetic resonance (NMR) spectroscopy has developed into a versatile and powerful analytical tool in multiple scientific disciplines. As a result of NMR's utility, the technique is included as a topic across the undergraduate chemistry curriculum. The American Chemical Society (ACS) makes the importance of this topic apparent in their requirements for approved undergraduate chemistry programs, with an NMR spectrometer listed as the only mandatory instrumentation (ACS Guidelines, 2015). NMR spectroscopy is typically taught in introductory organic chemistry courses, where it is generally considered difficult to both teach and learn because it requires an understanding of complex concepts such as spin–spin coupling and chemical equivalency that are not inherent to other chemistry topics taught at the introductory level. Problem solving in the form of 1H NMR spectral interpretation is also regularly included in the instruction of this topic given that 1H NMR spectroscopy has found the widest application among chemists (Gerothanassis et al., 2002). The necessity for problem-solving skills in addition to conceptual understanding further contributes to the difficulty of teaching and learning this topic (Angawi, 2014).Classroom instruction plays an integral role in students’ learning of basic principles of NMR spectroscopy and the practice of 1H NMR spectral interpretation. Most organic chemistry textbooks provide general guidelines for 1H NMR spectral interpretation, however these guidelines are insufficient for student learning (Angawi, 2014). As a result of the importance of this topic, undergraduate classroom instruction of NMR spectroscopy has received significant attention in chemistry education research literature. The vast majority of this attention has focused on the development of NMR spectroscopy laboratory experiments (Lorigan et al., 2001; Simpson et al., 2015; Wright, 2016) and instructional scaffolding strategies (Winschel et al., 2015). In addition, empirical studies have characterized successful and unsuccessful problem-solving approaches among interpreters of 1H NMR spectra in order to inform classroom instruction (Cartrette and Bodner, 2010; Topczewski et al., 2017). However, little is known about instructors’ knowledge for teaching 1H NMR spectroscopy despite the abundance of curricular ideas for teaching this topic. An understanding of this knowledge, including how it develops, is needed in order to inform instructor education and in turn improve classroom instruction.
Pedagogical content knowledge (PCK) is an integral component of teaching knowledge. This theoretical construct was conceptualized by Shulman (1987) and defined as teachers’ knowledge of the most useful and meaningful ways of transforming subject matter in order to make it comprehensible to learners. The most recent consensus model of PCK defines the construct as knowledge for teaching a particular topic, termed “reflection on action,” combined with teachers’ specific ways of acting on this knowledge, termed “reflection in action” (Gess-Newsome, 2015). A number of empirical studies have attempted to characterize and measure instructors’ PCK as a means of understanding their knowledge for teaching (Jüttner et al., 2013). Recognition that this theoretical construct has value for implementation in instructor training programs has further contributed to the increase in the number of studies aimed at its characterization (Kind, 2009; Mavhunga and Rollnick, 2013). Empirical studies have demonstrated that instructors’ PCK can be improved through training programs (Mavhunga and Rollnick, 2013) and that instructors’ PCK positively correlates with both instruction quality and student learning outcomes (Hill et al., 2005; Park et al., 2011). Characterizing instructors’ PCK thus serves as a means of understanding knowledge for teaching a topic and improving instructor education, instruction quality, and student learning outcomes.
This study aimed to investigate how teaching assistants (TAs) develop PCK in 1H NMR spectroscopy and the nature of their PCK in order to gain insight into instructors’ knowledge for teaching this topic. Such insight could then be used to improve instructor education, instruction quality, and student learning outcomes in this topic. More specifically, this study sought to address the following research questions:
(1) How do TAs’ content knowledge and teaching experience influence their development of PCK in 1H NMR spectroscopy?
(2) What is the nature of TAs’ PCK in 1H NMR spectroscopy?
Theoretical framework and background
Pedagogical content knowledge
Shulman (1986, 1987) originally described PCK as “subject matter knowledge for teaching,” or a teacher-specific type of knowledge that combines content and pedagogy and allows for the transformation of subject matter into a form comprehensible for learners. PCK is both personal and canonical; personal PCK develops through reflection on one's own practice, and canonical PCK forms through social means such as professional development (Smith and Banilower, 2015). PCK can be examined at the discipline-specific, subject-specific, and topic-specific levels. A teacher with discipline-specific PCK would have an understanding of pedagogical concepts and strategies for teaching in a particular discipline (e.g. science, art, history or mathematics), whereas a teacher with subject-specific PCK would have this understanding for a particular subject within a discipline (e.g. the subject of chemistry in the science discipline). At the topic-specific level, the PCK of a teacher will differ for the particular topic being taught. For example, a chemistry teacher will have a different understanding and approach for how to best teach chemical equilibrium versus particle theory (Veal and MaKinster, 2001). Moreover, identifying the ultimate “grain-size,” or domain-specificity, of PCK presents a challenge to researchers; Shulman (2015) made this uncertainty evident by questioning in his opening address at the PCK Summit if this domain encompasses a discipline, a field of practice, specific topics, or even certain problems within a discipline.The most recent conceptualization of PCK emphasizes the construct's topic-specific nature (Gess-Newsome, 2015). Instructors with topic-specific PCK (TS-PCK) are those who can appropriately transform their content knowledge in a topic into a form comprehensible for learners using five components of their teaching knowledge: (1) learners’ prior knowledge, including misconceptions; (2) curricular saliency (i.e. the specific content in a curriculum, the sequence in which it is presented, prerequisite knowledge, and the importance of teaching the content); (3) what makes the topic easy or difficult to understand; (4) representations, including powerful examples and analogies; and (5) conceptual teaching strategies (Mavhunga and Rollnick, 2013). A common approach for evaluating PCK has been to identify components that form PCK and then view the construct as an amalgamation of those components (Park and Oliver, 2008).
This study examines PCK at the topic-specific level. The topic-specific nature of TS-PCK aligns with constructivist theories of learning, which describe the growth of instructors’ understanding as a construction process specific to the content, students, and context in which the content is taught (Cochran, 1993). This alignment indicates that TS-PCK is an appropriate framework for evaluating how instructors develop teaching expertise for a particular topic. The model of TS-PCK also places emphasis on both content knowledge in a topic and the components that transform it, allowing for the development of teaching expertise to be more directly modeled.
Content knowledge
Shulman (1987) identified content knowledge (CK), often referred to as subject matter knowledge, as one of several knowledge bases for teaching. CK is necessary but not independently sufficient for forming PCK, however the exact relationship between CK and PCK remains unclear (Jüttner et al., 2013; Rollnick et al., 2017). CK of participants thus merits evaluation. CK for this study is aligned with Ausubel's theory of meaningful learning (Ausubel et al., 1978), which assumes that in order for meaningful learning to occur, the learner must have relevant prior knowledge, the new knowledge must relate to this prior knowledge in a meaningful way, and the learner must choose to integrate this new knowledge into existing prior knowledge.Research on TS-PCK in chemistry topics
Recent studies on PCK have contributed to an understanding of instructors’ TS-PCK for chemistry topics that contain a central problem-solving component, much like NMR spectroscopy. Problem solving is conceptualized in this study as completing a task that contains unfamiliar aspects and extends beyond a routine exercise for the problem solver (Bodner and Domin, 2000). Successful problem solving thus requires an integration of conceptual understanding rather than merely the application of an algorithm. In their investigation of TS-PCK relating to the mole, Rollnick et al. (2008) found that teachers often promoted algorithmic approaches to mathematical problem solving rather than conceptual understanding. Malcolm (2015) also suggested that instructors were quite capable of providing teaching strategies for stoichiometry problems potentially because of a reliance on algorithms rather than developing conceptual understanding relating to this topic. It is unclear if this reliance on algorithms while teaching is specific to stoichiometry and the mole or if it extends to other chemistry topics in which problem solving is a central component, namely NMR spectroscopy. Although NMR spectral interpretation is a type of nonmathematical problem solving involving the determination of spatial relationships (Cartrette and Bodner, 2010), instructional strategies that rely on algorithms may also extend to this topic. This would be a problematic teaching strategy among instructors given that a single 1H NMR spectrum has the potential to provide an abundance of information, yet there is no straightforward algorithm or general procedure for spectral interpretation (Angawi, 2014).A number of empirical studies on chemistry instructors’ PCK have also contributed to a developing understanding of how to cultivate knowledge for teaching chemistry topics. Mavhunga (2014) demonstrated that explicitly discussing a topic through the five components of TS-PCK and engaging with concepts in the topic improves teachers’ TS-PCK in that topic. The ability to use the five components of TS-PCK is then transferrable to other topics, but these components must be accompanied by successful engagement with the concepts of the new topic if TS-PCK in this new topic is to improve. By identifying the component most accessible to teachers, a possible leverage point can be established in this transfer process (Rollnick and Mavhunga, 2015). This finding aligns with that of Charalambous et al. (2011), who demonstrated that incorporating a high-leverage practice into a prospective teacher training program results in prospective teachers improving in this practice, given that they engage in active reflection. In an investigation of pre-service chemistry teachers’ TS-PCK relating to the particulate nature of matter, knowledge of curricular saliency was identified as the most accessible component to teachers (Rollnick and Mavhunga, 2015). This finding was deemed encouraging given that it provides a potential means of “incrementally building topic-specific professional knowledge across core chemistry and physics topics.” However, in a similar study investigating the TS-PCK of “novice unqualified graduate science teachers” in the particulate nature of matter, learner's prior knowledge was the most accessible component, whereas curricular saliency was much less accessible (Pitjeng, 2014). Learner's prior knowledge was also identified as the most accessible component in a study of pre-service teachers’ TS-PCK in stoichiometry, whereas curricular saliency was identified as the least accessible component (Malcolm, 2015). These findings call into question whether the leverage point for transfer is potentially dependent upon the training of the instructor or the specific topic. Additional investigations of PCK at the topic level are needed to address this uncertainty.
Methods
A mixed methods approach was used to characterize TS-PCK in 1H NMR spectroscopy among teaching assistants (TAs). As part of this approach, a questionnaire was designed to provide a measure of TAs’ CK and TS-PCK in 1H NMR spectroscopy. Responses to the TS-PCK component of the questionnaire were quantitatively transformed using a rubric and analyzed using the Rasch model (Bond and Fox, 2007). Responses were also qualitatively analyzed to gain a richer understanding of TS-PCK in this topic. Questions were validated using the Rasch model, evaluation by external experts, and cognitive interviews (Collins, 2003). The questionnaire was accompanied by a survey that assessed TA's teaching experience, interest, and background information.Participants
The study was performed at a large Midwestern university and consisted of 20 participants (16 graduate TAs enrolled in a doctoral program, two post-doctoral fellows, and two undergraduate TAs) with a range of teaching experience. Graduate and undergraduate TAs are formally referred to as graduate student instructors and undergraduate instructor assistants, respectively, at the university in which the study took place. TAs were selected as participants for two reasons. Firstly, TAs play a prominent role in undergraduate education at doctoral granting institutions (Dragisich et al., 2016). Understanding TAs’ knowledge for teaching is therefore critical for improving classroom instruction at large universities. Secondly, TAs commonly teach NMR spectroscopy at the institution in which the study took place. Participants with a range of teaching experience could thus be recruited in order to better understand how knowledge for teaching 1H NMR spectroscopy develops.TAs at this institution receive two days of formal training that primarily focuses on laboratory management. They also receive varying amounts of informal guidance, suggesting that participants likely possessed personal rather than canonical PCK (Smith and Banilower, 2015). Faculty members with extensive teaching experience in NMR spectroscopy were not included as participants in this study population because they likely develop knowledge for teaching that is distinct from that of TAs. This difference is due to the situated nature of expertise. In line with socio-cultural views of teacher learning, an expert is one who engages fully in social practices specific to the area of expertise (Kelly, 2006); because TAs do not engage in the same social practices of teaching as faculty members (e.g. determining learning objectives, selecting content to present throughout a course, etc.), TAs will develop teaching expertise that differs from that of faculty members. While faculty members’ knowledge for teaching NMR spectroscopy is of interest to the authors, it is beyond the scope of this study and will be the focus of future work.
NMR spectroscopy is taught in Organic Chemistry I lecture and Organic Chemistry II laboratory at this university. NMR spectroscopy is introduced in a limited fashion in Organic Chemistry I lecture, where only the concept of distinct chemical environments in 13C and 1H NMR spectroscopy is covered. TAs serve as discussion leaders for the recitation component of this lecture. NMR spectroscopy is taught more comprehensively in Organic Chemistry II laboratory, where spectral interpretation and more complex concepts of spin–spin coupling, chemical shift, and topicity effects are covered. Participants had a range of teaching experience in other chemistry courses. Two additional individuals not included in the 20 participants completed cognitive interviews during initial question piloting. All individuals voluntarily consented to participate in the study and IRB approval was obtained.
Questionnaire design
A questionnaire was designed to provide an inferential measure of TAs’ CK and TS-PCK in 1H NMR spectroscopy. A questionnaire blueprint similar to that of Jüttner et al. (2013) was developed to assist with CK and PCK question design (Table 1). CK and PCK questions were written by adapting problems from an organic chemistry textbook (Bruice, 2011) and consulting with a faculty member who has over ten years of experience teaching organic chemistry. Given that organic chemistry curricula do not vary significantly among instructors or institutions (Raker et al., 2015), PCK questions thus had the potential to elicit insight into knowledge for teaching NMR spectroscopy that may be broadly applicable to instruction of this topic.| Type of CK | CK question |
|---|---|
| Procedural | CKQ1, Determine structure from spectrum. |
| CKQ2, Determine structure from spectrum. | |
| Declarative | CKQ3, Which sets of hydrogen atoms are equivalent? |
| CKQ4, Which sets of molecules are distinguishable using 1H NMR? | |
| CKQ5, How many 1H NMR signals does the molecule produce? | |
| Component of PCK | PCK question |
|---|---|
| What makes a topic difficult | PCKQ1, What do students find most difficult about NMR spectral interpretation? |
| What makes a topic difficult and teaching strategies | PCKQ2, What would a student find difficult about determining a structure from this spectrum, and how would you help them interpret the spectrum? |
| PCKQ3, Did the student correctly elucidate a structure from this spectrum, what (if anything) created difficulty, and how would you help them interpret the spectrum? | |
| PCKQ4, Did the student correctly determine equivalent hydrogen atoms, what (if anything) created difficulty, and how would you help them determine the correct answer? | |
A second tier was included in PCK questions that asked TAs to identify whether their experience as a teacher, researcher, and/or student informed their response. Participants were instructed to select as many options as applicable. This second tier was included to determine if participants drew on direct teaching experience and not just reasoning from experience as a student. Responses to the second tier also provided insight into the collective research experience in NMR spectroscopy among the study population. The response frequencies to this second tier are provided in Appendix 1 (Table 12). The questionnaire was piloted with two content experts who were not part of the project team using cognitive interviews (Collins, 2003) to investigate whether all the questions were interpreted as intended. Content experts were graduate students with teaching experience in organic chemistry and research experience in organic chemistry and chemistry education. The content experts then discussed all questions with one author, and revisions were made in accordance with their suggestions in order to improve question clarity. After the initial pilot, two study participants participated in cognitive interviews to evaluate the final version of the questionnaire. These interviews revealed that one final PCK question was not interpreted as intended; this PCK question was omitted from subsequent analysis.
The final version of the questionnaire contained five CK questions and four PCK questions. The complete set of CK and PCK questions are provided in Appendices 2 and 3, respectively. CK questions were placed at the beginning of the questionnaire, followed by PCK questions; this was done to prevent TAs from using a proton chemical shift table provided in PCK questions to answer CK questions. Experts reported only slight difficulty in responding to the CK component during question piloting, so a 25 minute time limit was imposed with the final version to increase difficulty and reduce overall time for participation. No time limit was imposed on the PCK component so that TAs could provide responses that completely captured their thinking. Participants took approximately one hour to respond to the PCK component.
The questionnaire blueprint (Table 1) identifies the components of CK or PCK that each particular question targeted. CK questions were written to assess TAs’ procedural and declarative knowledge of 1H NMR spectroscopy (Alexander et al., 1991). Procedural CK questions required TAs to elucidate the molecular structure corresponding to a provided 1H NMR spectrum and molecular formula. 1H NMR spectra for the CK component were retrieved from the Spectral Database for Organic Compounds (SDBS, 1997). Declarative CK questions assessed TAs’ knowledge of proton equivalency and spin–spin coupling. PCK questions were written to probe two components of PCK: ‘what makes a topic difficult’ and ‘teaching strategies’ (Mavhunga and Rollnick, 2013). 1H NMR spectra for the PCK component were generated using ChemDraw (ChemDraw Professional 16.0, 2017). For PCK questions that involved spectral interpretation, the faculty member consulted during initial question drafting provided perspectives on what spectral features most commonly make interpretation difficult for students. Although TAs and faculty members likely have distinct knowledge for teaching, insight into the most common difficult features allowed for the design of questions that would most likely elicit TAs’ PCK. The content experts also indicated that all PCK questions were appropriate for assessing TAs’ knowledge for teaching.
Measuring particular components of PCK has been shown to provide a reliable measure of overall PCK (Rowan et al., 2001), so the questionnaire was designed to probe only two of the five components of PCK. Researchers hold different conceptualizations of the components that contribute to PCK, however there is consensus that knowledge of students’ understanding and knowledge of instructional strategies are integral to the construct (Schmelzing et al., 2013). ‘What makes a topic difficult’ and ‘teaching strategies’ were selected because they align with this essential knowledge. In addition, the overall quality of PCK depends upon both the quality of individual components and the coherence among components, and knowledge of students’ understanding and knowledge of instructional strategies are central in the integration of multiple PCK components (Park and Chen, 2012). By providing participants with questions that best allowed them to integrate multiple components of PCK, the quality of their PCK could be more effectively measured.
Additional data for this study included audiotaped cognitive interviews with two content experts and two study participants, as well as responses to survey questions that characterized TA teaching experience, interest in teaching, and additional background information.
Data analysis
PCK responses were scored on a 0–4 point scale using separate rubrics for each targeted PCK component (Table 2). Responses ranged from incorrect (0) to exemplary (4). The rubric used to score ‘what makes a topic difficult’ responses was similar to that of Mavhunga (2016) and Park and Oliver (2008). Developing (3) and exemplary (4) responses were those that incorporated either one or two components of PCK, respectively. The total frequency of each PCK component in responses was also determined.| Score | What makes a topic difficult | Teaching strategies |
|---|---|---|
| 0 Incorrect | – Provides incorrect explanation | – Provides incorrect explanation |
| 1 Limited | – Identifies difficult aspect | – Provides problem-solving method |
| – Provides no reasoning | – Does not relate method to problem | |
| 2 Basic | – Identifies difficult aspect | – Provides problem-solving method |
| – Provides broad and generic reasoning | – Relates method to problem | |
| 3 Developing | – Identifies difficult aspect |
– Uses interactive teaching, e.g. questioning to probe or promote students’ understanding
or – Uses illustrations or models during explanations |
| – Provides reasoning relating to one PCK component: | ||
| Learner's prior knowledge | ||
| Conceptual teaching strategies | ||
| Representations (examples or models) | ||
| Curricular saliency | ||
| 4 Exemplary | – Identifies difficult aspect | – Recognizes learners’ prior knowledge |
| – Provides reasoning relating to two or more | ||
| PCK components |
The rubric used to score ‘teaching strategies’ responses was similar to that of Hale et al. (2016). Developing (3) responses incorporated either interactive teaching or the use of representations during explanations. “Interactive teaching” on this rubric was consistent with the definition described by Chin (2007). Two coders discussed and revised operational definitions in the rubric until 96% agreement was reached. A third coder then scored a subset of questionnaire responses (15%) using the finalized rubric, and an acceptable Cohen's kappa (0.736) was achieved (Landis and Koch, 1977). For PCK items that targeted both PCK components, ‘teaching strategies’ and ‘what makes a topic difficult’ scores were averaged. CK responses were scored based on a correct or incorrect basis. Exemplars corresponding to each rubric and PCK question are included in Appendix 1 (Tables 5–11).
Raw CK and TS-PCK scores were subjected to Rasch analysis using Winsteps software (Linacre, 2017a). The Rasch model places person ability and item difficulty on the same scale in logit units. A logit is defined as the logarithmic transformation of the odds of success (Bond and Fox, 2007). The model sets the mean item difficulty to zero logit units, meaning that an item of average difficulty would have a logit unit equal to zero. The unidimensionality of the model provides an inferential measure of a person's overall ability relating to a single latent variable, in this instance, CK or TS-PCK (Bond and Fox, 2007). The Rasch model also provides a measure of the validity of the results through reliability estimates and model fit statistics.
Results
A questionnaire was developed to provide an inferential measure of teaching assistants’ CK and TS-PCK in 1H NMR spectroscopy. The results were validated through several processes: face validity (Haynes et al., 1995) of the questionnaire was established through consultation with external experts and construct validity (Cronbach and Meehl, 1955) through cognitive interviews and analysis using the Rasch model. The questionnaire was administered to 20 TAs, and their responses were evaluated both quantitatively and qualitatively.Questionnaire development and Rasch model validity
The questionnaire for measuring CK and TS-PCK was validated in part using the Rasch model. Item reliability, a measure of the extent to which items represent a range of difficulty relating to a single variable, and person reliability, a measure of whether the questionnaire appropriately discriminates across the ability range of participants, were used as reliability indices (Bond and Fox, 2007). Rasch measures produced acceptable person and item reliability indices for the PCK component, as well as an acceptable item reliability for the CK component. For the PCK component of the questionnaire, item reliability was 0.96 and person reliability was 0.78. In the case of the CK component, item reliability was 0.81 and person reliability was 0.47. Acceptable item and person reliability indices for the CK and PCK components were similar to those found by Mavhunga and Rollnick (2013) and Jüttner et al. (2013). Person reliability for the CK component was low but similar to that found by Hale et al. (2016). This low reliability can be explained by the high CK of TAs. CK questions were derived from an undergraduate organic chemistry textbook (Bruice, 2011), so most TAs were able to perform well on these questions and, in effect, decrease the questionnaire's capability to discriminate among CK levels.Fit statistics were used to assess the questionnaire's level of productive measurement. For all CK and PCK items, MNSQ < 1.5 and/or t ≤ |2|, indicating that items were productive for measurement (Linacre, 2017a). For all persons on the CK component, MNSQ < 1.5 and/or t ≤ |2|, confirming that all participants fit the Rasch model. For 19 out of 20 person measures on the PCK component, MNSQ < 1.5 and/or t ≤ |2|; this was acceptable given that 5% of people are expected to misfit the model by chance (Linacre, 2017b). These acceptable fit statistics further validate that the questionnaire reliably measures CK and TS-PCK.
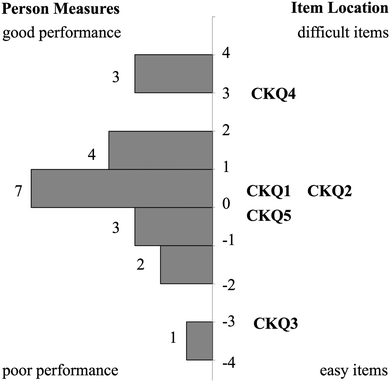
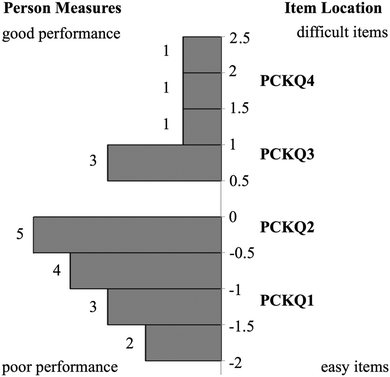
Relationship between CK and PCK person measures
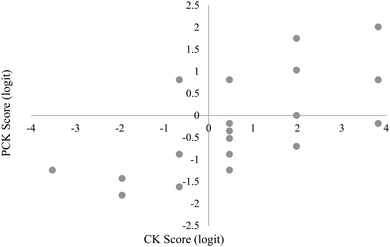
The relative placement of person ability and item difficulty determined through Rasch analysis can be depicted using an item-person map (Fig. 1 and 2). A person's location on this map indicates the person's ability to correctly respond to questions of a given difficulty. For example, if a person and item have the same logit measure, then the person has a 50% chance of answering a similar item correctly. This person would have greater than a 50% chance of sufficiently answering items of lower difficulty (i.e. lower logit value) and lower than a 50% chance of correctly answering items of higher difficulty (i.e. higher logit value). CK and PCK person measures generated by the Rasch model may provide insight into how TS-PCK in 1H NMR spectroscopy develops. According to the item-person map for the CK component of the questionnaire (Fig. 1), TAs had relatively high CK in 1H NMR spectroscopy, with the mean person measure equal to 0.66 logits. TAs had comparatively low TS-PCK in 1H NMR spectroscopy, with the mean person measure equal to −0.21 logits (Fig. 2). A small number of TAs were able to score well above the −0.21 logit average measure, suggesting that these TAs may represent the upper anchor of TA teaching expertise for this topic. The relatively low level of TS-PCK compared to CK aligns with the notion that CK is necessary but not sufficient for the development of PCK (Pitjeng, 2014). This finding was also similar to that of Hale et al. (2016).CK and PCK person measures are depicted on a scatterplot in Fig. 3. A strong and significant positive correlation was found between CK and PCK (Table 3), which also aligns with the notion that CK is necessary for the development of PCK. This result is consistent with those of Hale and Jüttner (Jüttner et al., 2013; Hale et al., 2016) and further validates that the questionnaire measured what was conceptualized as TS-PCK. Data points in the lower right quadrant correspond to TAs with high CK scores but low PCK scores; this observation supports the general agreement that CK is necessary but not sufficient for the development of PCK. One data point unexpectedly populates the upper left quadrant corresponding to relatively low CK and high PCK. This could be due to this participant not performing well in timed test-taking situations.
| Indices | Content knowledge | Pedagogical content knowledge |
|---|---|---|
| * indicates p (two-tailed) < 0.05, ** indicates p (two-tailed) < 0.01. | ||
| Content knowledge | 1 | 0.670** |
| Pedagogical content knowledge | 0.670** | 1 |
| Number of chemistry courses taught | −0.153 | −0.308 |
| Number of terms teaching Organic II lab | −0.075 | −0.073 |
| Relative teaching experience (number of terms teaching Organic II lab divided by number of chemistry courses taught) | 0.278 | 0.490* |
| Terms since last teaching Organic II lab | −0.397 | −0.432 |
| Teaching interest | 0.367 | 0.424 |
| Organic sub-discipline | 0.148 | −0.217 |
Dependence of PCK on CK
The Rasch model provided an ordering of CK and PCK questions from least to most difficult that may also provide insight into how TAs develop TS-PCK in 1H NMR spectroscopy. This ordering, termed a difficulty hierarchy (Linacre, 2004), is depicted in each respective item-person map (Fig. 1 and 2). A comparison of CK and PCK difficulty hierarchies reveals that CK questions that targeted procedural knowledge align in difficulty with PCK questions that targeted procedural knowledge. For example, CKQ1 and CKQ2 (Fig. 1) and PCKQ2 and PCKQ3 (Fig. 2) were all of intermediate difficulty, and all assessed procedural knowledge involved in elucidating a structure from a spectrum. Additionally, CK questions that targeted declarative knowledge also align in difficulty with PCK questions that targeted declarative knowledge. CKQ5 and PCKQ4 were of greatest difficulty, and both assessed declarative knowledge relating to the determination of topicity in complex scenarios. The similarity in CK and PCK difficulty hierarchies suggests that TS-PCK depends on CK in that topic, which is consistent with PCK theory, but also that the development of PCK may be affected specifically by the declarative or procedural CK required for a particular sub-topic or problem. This alignment between CK and PCK questions assessing similar procedural and declarative knowledge contrasts with the findings of Jüttner et al. (2013), who found no alignment between CK and PCK questions that targeted declarative and procedural knowledge on an instrument designed to assess PCK in four biology topics. This alignment between declarative and procedural questions may therefore only be observable when examining PCK at the topic-specific level for a single topic.Relationship between PCK and teaching experience
A significant positive correlation was found between PCK and relative teaching experience (Table 3). We define relative teaching experience as the number of times a TA taught Organic Chemistry II laboratory relative to the total number of chemistry courses the TA taught. For example, if a TA taught Organic Chemistry II laboratory once and General Chemistry laboratory twice, relative teaching experience would equal 0.33. As previously noted, NMR spectroscopy is only taught in Organic Chemistry I lecture and Organic Chemistry II laboratory at the university in which the study took place, and NMR spectroscopy is introduced in a very limited manner in Organic Chemistry I lecture, where only the concept of distinct chemical environments in 13C and 1H NMR spectroscopy is covered. NMR spectroscopy is taught more comprehensively in Organic Chemistry II laboratory, and this is the only course in which TAs would teach spectral interpretation and the inherent concepts of spin–spin coupling and chemical shift that must be incorporated into problem solving. The relationship between PCK and relative teaching experience suggests that TAs develop TS-PCK in 1H NMR spectroscopy by teaching chemistry courses in which NMR is explicitly taught. This further supports the consensus that PCK is topic-specific in nature (Gess-Newsome, 2015) and further validates that the questionnaire measures TS-PCK.No significant relationship was found between PCK and total chemistry teaching experience or teaching experience in Organic Chemistry II laboratory. This finding was not consistent with that of Hale et al. (2016) who found that overall teaching experience was correlated for TAs with TS-PCK in thin layer chromatography. However, this result may be attributed to the sample size of the study; within this small sample size were participants who had previous taught Organic Chemistry II laboratory but had also taught many other courses in which 1H NMR spectroscopy is not explicitly taught. In some cases, TAs had experience teaching Organic Chemistry II laboratory, but a substantial time lapse had occurred between that experience and participation in the study. A potential inability among these participants to recall knowledge for teaching this particular topic may have contributed to the insignificant relationship between PCK and overall teaching experience. Further, chromatography is a general approach that TA's may have encountered in other courses and contexts, and such experience may be more easily translated to specific chromatographic techniques such as TLC. Chromatography is also conceptually more tractable than NMR. Finally, we also found no significant relationship between PCK and teaching interest, terms since last teaching, or research sub-discipline.
Identifying a leverage point for transfer of TS-PCK
Analysis of responses to PCK questions may provide insight into the nature of TS-PCK in 1H NMR spectroscopy. TAs that demonstrated either “developing” or “exemplary” PCK on ‘what makes a topic difficult’ questions primarily did so by incorporating an understanding of learners’ prior knowledge into their responses. The frequencies of PCK components incorporated in “developing” or “exemplary” ‘what makes a topic difficult’ responses are depicted in Table 4. An understanding of curricular saliency, representations, and conceptual teaching strategies were present in responses to ‘what makes a topic difficult’ questions, however their frequencies were much lower (Table 4). The prevalence of this incorporation suggests that TAs found learners’ prior knowledge to be a relatively accessible component of PCK as opposed to other components. Rollnick and Mavhunga (2015) suggested that by identifying the component most accessible to instructors, a possible leverage point can be established in order to transfer the ability to use the five components of PCK to other topics. The accessibility of learners’ prior knowledge contrasts with the findings of Rollnick and Mavhunga (2015), who identified curricular saliency as being most accessible to pre-service chemistry teachers when investigating TS-PCK in the particulate nature of matter. However, it is consistent with those of Malcolm (2015), who identified learners’ prior knowledge as most accessible to pre-service teachers when investigating TS-PCK in stoichiometry. This alignment suggests that the potential anchoring component may depend upon the particular topic. Given that both stoichiometry and NMR include elements of problem solving, this difference may be attributed to the particular nature of the topics. Another possibility may be the nature of the study population. Pitjeng (2014) identified learners’ prior knowledge as the most accessible component among “novice unqualified graduate science teachers” developing TS-PCK in the particulate nature of matter. This agreement suggests that learners’ prior knowledge may be the most accessible component of PCK among instructors without extensive professional development in teaching, implying that the anchoring component may also depend on instructors’ training in addition to the particular topic.| PCK Component | Learners’ prior knowledge | Curricular saliency | Representations | Conceptual teaching strategies |
|---|---|---|---|---|
| Frequency | 24 | 4 | 4 | 1 |
Teaching strategies of TAs
For ‘teaching strategies’ PCK questions, TAs most commonly described interactive teaching strategies (Chin, 2007) involving questioning to probe or promote students’ understanding and the use of drawings or models during explanations. Of the 60 ‘teaching strategies’ responses, 23 employed interactive techniques or the use of representations during explanations. The frequencies of responses that described a teaching strategy related to the problem (11), a strategy unrelated to the problem (9), or a strategy based on incorrect CK (11) were much lower in comparison. The use of algorithms did not dominate TAs’ teaching strategies, with only seven out of 60 responses providing a simplified step-by-step problem-solving approach. This result contrasts with findings of other investigations on TS-PCK in chemistry topics with a central problem-solving component (Rollnick et al., 2008; Malcolm, 2015).Problem-specificity of PCK
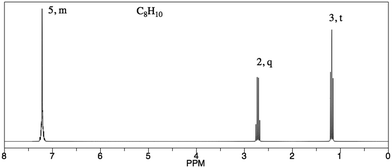
Analysis of PCKQ2 and PCKQ3 may provide insight into both the “grain-size” of PCK and the nature of TAs’ TS-PCK in 1H NMR spectroscopy. These questions were of similar problem type (i.e. determine ‘what makes a topic difficult’ and ‘teaching strategies’ for a problem where a student must determine a structure from a spectrum), yet they had different difficulty levels as depicted in the PCK item-person map (Fig. 2). For the less difficult item (PCKQ2), TAs readily identified a difficult feature and incorporated an understanding of learners’ prior knowledge into their responses. Thirteen out of 20 TAs identified a difficult feature on PCKQ2. TAs that identified a difficult feature mostly attributed difficulty to students not understanding that peaks in different chemical environments may potentially overlap. These observations align with what an experienced instructor of the course described as student difficulties specific to this problem during initial PCK item drafting. Many TAs also noted students’ rigidity and heavy reliance on the proton chemical shift table as a heuristic. Participants who demonstrated an “exemplary” response typically recognized this misconception and also incorporated another component of PCK. In the following response, this second component was an understanding of curricular saliency, in particular knowledge of the content presented in the curriculum:“A large difficulty for the student lies in the integration of the aromatic hydrogens. We teach them that they are groups of different hydrogens depending on their aromatic position ; however, it is now slightly confusing because those are now all grouped in one peak. An additional difficulty may lie in the reasoning for the downshift of the peak labeled ‘2, quartet’ as it does not exactly align with the NMR [chemical shift] chart.” – Participant One
The majority of participants also demonstrated either “developing” or “exemplary” ‘teaching strategies’ responses on PCKQ2. Seven out of 20 TAs demonstrated a “developing” transformation of CK to PCK by integrating interactive teaching into their teaching strategy, and four out of 20 TAs demonstrated an “exemplary” transformation of CK to PCK by incorporating an understanding of learners’ prior knowledge into their ‘teaching strategy’ response:
“The peak at 2.75 [ppm] would most likely be causing issues. The student probably sees a peak at 7.2 [ppm] and looks at the table and assigns it as an aromatic proton. The student looks at the 1.2 ppm peak and assigns it as alkyl. But the peak at 2.75 [ppm] doesn’t match anything on the table. They would tell me it is in the range for an alcohol peak or an amine, but the structure doesn’t contain O or N. I would first ask them to describe what kinds of protons account for the chemical shifts in the spectra. They would give me the answer [above], then I would talk them through how inductive effects can affect chemical shift. Similar to how an electronegative atom deshields protons adjacent to it, the aromatic ring can be thought of as electronegative, and pulls electrons away from adjacent protons. This means that even though the benzylic protons are alkyl, they can appear at a higher ppm than normal.” – Participant Two
TAs with low PCK scores often advocated for use of the proton chemical shift table as a heuristic and routinely suggested an algorithmic approach to problem solving. These TAs did not demonstrate knowledge of students’ rigidity while problem solving:
“1. Calculate degree of unsaturation. 2. Using chemical shift table + integration, identify likely functional groups containing [hydrogen atoms]. 3. Begin drawing possible structures, keep formula, symmetry (# of signals), + degree of unsaturation in mind. 4. Identify correct structure from your possibilities, making sure splitting agrees with assignment.” – Participant Three
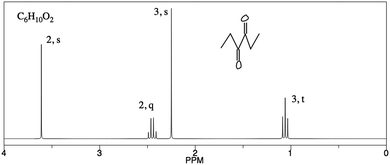
PCKQ3 was more difficult for TAs. Of the 20 responses to PCKQ3, only five identified a difficult feature. This problem involved elucidating the structure of 2,4-hexanedione, the methylene group of which has a chemical shift much further downfield than a chemical shift table would indicate. This problem-specific difficult feature was also identified by an experienced instructor of the course during PCK item drafting. No TA identified this particular feature, and TAs that did identify a feature attributed student difficulty to the student not understanding splitting patterns. Cognitive interviews suggested that among TAs who did not identify a challenging aspect, a difficult feature was not apparent. Failure to identify a feature was therefore not due to a misreading of the question or fatigue. Only one TA incorporated an understanding of learners’ prior knowledge into a teaching strategy. Notably, this was one of the four TAs who did so on PCKQ2:
“The student knew that the structure contained an ethyl group, but they should have realized that the structure they drew cannot account for the two singlets. In this case, I would suggest a guess and check approach. Just starting drawing structures, keeping the ethyl group but moving the other carbons around to get different connectivity. Keep changing the structure until all the NMR features are accounted for. I find that to be generally a good approach for me, as well as the students I have taught.” – Participant Two
The number of TAs who incorporated interactive teaching into their teaching strategy remained relatively unchanged from PCKQ2, with 11 out of 20 TAs able to make this incorporation on PCKQ3 compared to seven out of 20 on PCKQ2. Out of the four TAs who were able to incorporate learners’ prior knowledge into their teaching strategy on PCKQ2 but not PCKQ3, three still described interactive teaching strategies on PCKQ3. This relatively unchanged number of TAs using interactive teaching strategies, combined with the large decrease in the number of TAs able to identify a difficult feature on PCKQ3, suggests that TAs’ use of interactive teaching strategies is not strongly influenced by their understanding of what makes a problem difficult. These results also suggest that as TAs struggle to identify what makes a particular problem difficult, they also struggle to incorporate learners’ prior knowledge into their teaching strategy for that problem. The difference in ability to (1) recognize what would make different problems of an identical type (i.e. determine structure from spectrum) difficult for a student and (2) provide exemplary teaching strategies for these problems implies that TAs’ PCK may be specific to certain problems in addition to certain topics.
Discussion
A questionnaire was developed to provide an inferential measure of CK and TS-PCK in 1H NMR spectroscopy. The questionnaire was administered to TAs with a range of teaching experience, and their responses provided a means of understanding how TAs develop TS-PCK in 1H NMR spectroscopy, the nature of this TS-PCK, and the nature of PCK more broadly. Multiple findings emerged from the analysis of questionnaire responses. First, CK in NMR spectroscopy was significantly correlated with PCK in this topic, further supporting the notion that CK is necessary for the development of PCK. In addition, CK and PCK questions had similar difficulty hierarchies, with the most difficult question on both components targeting similar declarative knowledge and questions of intermediate difficulty on both components targeting similar procedural knowledge. This alignment suggests that the development of PCK may also depend upon the declarative or procedural CK required for a specific sub-topic or problem.Second, TAs’ TS-PCK in 1H NMR spectroscopy was significantly correlated with relative teaching experience. This correlation suggests that knowledge for teaching 1H NMR spectroscopy develops through practice teaching this topic, and that teaching additional courses that do not involve NMR spectroscopy may not contribute to the formation of TS-PCK in this topic. This correlation further supports the general agreement that PCK is topic-specific in nature. Practice teaching this topic may provide TAs with greater opportunity to engage in pedagogical reasoning (Shulman, 1987), a cyclic process that involves comprehension and transformation of the subject matter, instruction, evaluation, and reflection. Engagement in this process through practice teaching may then serve as the means by which TAs develop TS-PCK.
A third finding is that while TAs’ TS-PCK in 1H NMR spectroscopy was relatively low, they were not reliant on algorithmic approaches to problem solving and routinely described interactive teaching strategies. This result suggests that algorithmic approaches to problem solving may not be a significant component of practices for teaching NMR spectroscopy. Additionally, TAs most commonly demonstrated an understanding of learners’ prior knowledge relative to other components of PCK in their responses to ‘what makes a topic difficult’ questions. Given that TAs found this component of PCK relatively accessible when teaching NMR spectroscopy, this component may be a possible leverage point that allows TAs to transfer their ability to use PCK components to other topics. The accessibility of learners’ prior knowledge also suggests that discussing NMR spectroscopy through this component of PCK may serve as a way to initially cultivate knowledge for teaching this topic.
Lastly, TAs demonstrated a considerable difference in their ability to provide sufficient responses for PCKQ2 and PCKQ3, both questions that assessed procedural knowledge and involved elucidating a structure from a spectrum. The difference in ability to (1) recognize what would make different problems of an identical type difficult for students and (2) provide exemplary teaching strategies for each problem suggests that PCK may be specific to certain problems in addition to certain topics.
Conclusions
The questionnaire reported here provided an inferential measure of CK and PCK in 1H NMR spectroscopy, and it provided a means of investigating knowledge for teaching 1H NMR spectroscopy. Several significant findings emerged from this investigation. TAs with greater relative teaching experience in NMR spectroscopy were found to have higher levels of PCK in this topic, reinforcing the topic-specific nature of PCK. Results from the quantitative and qualitative analysis of responses to the questionnaire also provide evidence that the development of PCK is dependent upon CK required for a specific sub-topic or problem and that TAs develop PCK for specific types of problems in addition to specific topics. These results suggest that the domain-specificity of PCK may extend to the problem level.Limitations
PCK is difficult to measure because it exists both internally in the mind of a teacher and as an external construct. ‘Materials-based items,’ or questions that incorporate materials used in the classroom (e.g. worksheets completed by students or lesson plans), have been shown to reliably assess PCK (Carlson, 1990). The majority of short-answer PCK questions included in this questionnaire are a type of ‘material-based item’ and are thus appropriate for evaluating TAs’ PCK. However, this evaluation is still limited given that short answer responses only reflect thinking or teaching approaches that TAs choose to report and not necessarily what they might think or do in the classroom. To add to this limitation, scorers were required to make inferences about the intended meaning of TAs’ sometimes ambiguous responses. In these instances, responses were regularly given the higher potential score. Additionally, the relatively small sample size of the study was also a limitation that may have resulted in the insignificant correlations between PCK and total chemistry teaching experience or teaching experience in Organic Chemistry II laboratory. However, as indicated by the person reliability index for the PCK component of the questionnaire, participants demonstrated a range of teaching ability; the study population was therefore representative in that regard.Implications
A number of studies have attempted to characterize PCK in order to incorporate the construct into instructor training programs and in turn improve instructor education and student learning outcomes (Hill et al., 2008; Mavhunga and Rollnick, 2013). This study provided additional insight into the nature of PCK that may facilitate such an incorporation; our findings suggest that PCK may extend to the problem or sub-topic level, implying that instructors may first develop knowledge for teaching problems or sub-topics and this knowledge may then contribute to their knowledge for teaching a topic. In order to facilitate instructors’ development of knowledge for teaching, training programs may therefore need to initially focus on the development of knowledge for teaching problems or sub-topics before aiming to cultivate knowledge for teaching a topic. In addition, the accessibility of learners’ prior knowledge among participants suggests that this component of PCK may serve as a leverage point to begin building knowledge for teaching among either TAs or instructors of 1H NMR spectroscopy. Lastly, our results suggest that knowledge for teaching 1H NMR spectroscopy develops through practice and that teaching additional courses in which this topic is not included may fail to contribute to the development of TS-PCK. This finding implies that TAs should regularly teach the same course whenever possible in order to improve classroom instruction of 1H NMR spectroscopy and other topics, though this may pose a challenge to chemistry departments at doctoral granting institutions.Conflicts of interest
There are no conflicts to declare.Appendix 1: exemplars and second tier response frequencies
Tables 5–11: PCK question exemplars| What makes a topic difficult score | Participant response | |
|---|---|---|
| 1 Limited | – Identifies difficult aspect | “Stereochemistry – especially equivalent hydrogens on rotatable bonds versus non-rotatable bonds.” |
| – Provides no reasoning | ||
| 2 Basic | – Identifies difficult aspect | “Gathering all the information necessary from what is given. What I mean by this is getting students to realize the spectrum not only gives them information but also the molecular formula in terms of conjugation. This also goes into looking at the number of [hydrogen atoms], the splitting, as well as the shift.” |
| – Provides broad and generic reasoning | ||
| 3 Developing | – Identifies difficult aspect | “In my experience, most students had a hard time interpreting results/spectral data that didn’t follow exactly the parameters they were taught – many relied heavily on the spectral tables in their notebooks, using it as a crutch. They could not fathom that a methyl group might be higher than 1.6 ppm if it was between an oxygen [atom] and a double bond.” |
| – Provides reasoning relating to one PCK component: | ||
| Learner's prior knowledge | ||
| Conceptual teaching strategies | ||
| Representations (examples or models) | ||
| Curricular saliency | ||
| 4 Exemplary | – Identifies difficult aspect | “The most challenging aspects for introductory organic chemistry students, I have found have been the concept of chemical shifts and how it is additive and not A = B (look at chart and find exact answer) every time… Students struggle to grasp that they may have to apply what they know about electronegativity and hybridization to figuring out how chemical shifts can be altered/shifted differently than the values given to them in the chart. Another aspect is the concept of [stereochemistry]/topicity which is taught briefly but not very emphasized. Students often have trouble visualizing in 3D which leads to confusion about how [stereochemistry] can affect equivalence of [hydrogen atoms].” |
| – Provides reasoning relating to two or more | ||
| PCK components | ||
| What makes a topic difficult score | Participant response | |
|---|---|---|
| 1 Limited | – Identifies difficult aspect | “The broad, downfield multiplet compared to the distinct quartet, triplet.” |
| – Provides no reasoning | ||
| 2 Basic | – Identifies difficult aspect | “I think the initial challenge is that there are only three different proton signals even though there are 10 [hydrogen atoms].” |
| – Provides broad and generic reasoning | ||
| 3 Developing | – Identifies difficult aspect | “A large difficulty for the student lies in the integration of the aromatic hydrogens. We teach them that they are groups of different hydrogens depending on their aromatic position; however, it is now slightly confusing because those are now all grouped in 1 peak.” |
| – Provides reasoning relating to one PCK component: | ||
| Learner's prior knowledge | ||
| Conceptual teaching strategies | ||
| Representations (examples or models) | ||
| Curricular saliency | ||
| 4 Exemplary | – Identifies difficult aspect | No examples. |
| – Provides reasoning relating to two or more | ||
| PCK components | ||
| Teaching strategies score | Participant response | |
|---|---|---|
| 1 Limited | – Provides problem-solving method | “1. Calculate degree of unsaturation. 2. Using chemical shift table + integration, identify likely functional groups containing [hydrogen atoms]. 3. Begin drawing possible structures, keep formula, symmetry (# of signals), + degree of unsaturation in mind. 4. Identify correct structure from your possibilities, making sure splitting agrees with assignment.” |
| – Does not relate method to problem | ||
| 2 Basic | – Provides problem-solving method | “First we should be able to see we have [a] benzene ring in our structure (5 proton[s], shift ∼ 7.3 [ppm]). Then we see the other peaks have two [hydrogen atoms] and three [hydrogen atoms]. If we see the shift of methyl attached to benzene, it's ∼2.3 [ppm] and methyl attached to alkyl it's ∼0.9 [ppm]. Therefore we can assume that 2 [hydrogen atoms are] attached to carbon next to benzene, and 3 [hydrogen atoms are] attached to carbon next to alkyl. The quartet makes sense for [the two hydrogen atoms] and triplet makes sense for [the three hydrogen atoms] in this structure.” |
| – Relates method to problem | ||
| 3 Developing | – Uses interactive teaching, e.g. questioning to probe or promote students’ understanding | “I would start by telling them to figure out what types of protons may be present (i.e. aromatic, alkane, alkene, etc.) I would then ask what the triplet, quartet, and multiplets mean in terms of what other protons are around each set of protons. From there, they would hopefully connect that the t and q are next to each other + the multiplet isn’t interacting w/ these problems. After that, they could piece together there is an aromatic ring w/ an alkane chain off it.” |
| or | ||
| – Uses illustrations or models during explanations | ||
| 4 Exemplary | – Recognizes learners’ prior knowledge | “I’d tell the student to first identify the degree of unsaturation. This will suggest a benzene ring. Then identify the diagnostic ethyl. They should then arrive at ethyl benzene. We could then try to rationalize the ‘single’ aromatic peak. Since they observe that it is a multiplet, then they can rationalize that it is clearly not a single peak, but likely multiple peaks that come out very near one another w/ splitting. This peak is likely what they expect, a combination of a doublet: 2 × triplet. So why are they on top of one another? I would then explain that the electron donorability of an ethyl is comparable to a [hydrogen atom]. Therefore, the [hydrogen] atoms on the ring are very minimally effected by the electronic change, and therefore their peaks don’t shift appreciably. I may then find an example spectrum of maybe analine or something and show that a greater electronic change will make the resonance move apart.” |
| What makes a topic difficult score | Participant response | |
|---|---|---|
| 1 Limited | – Identifies difficult aspect | “It is not the correct structure. The difficulty lies within the integration and splitting patterns.” |
| – Provides no reasoning | ||
| 2 Basic | – Identifies difficult aspect | “I would expect that the presence of 2 singlets would be confusing because that adds to the complexity of the molecule + making sure there are protons not near these problems.” |
| – Provides broad and generic reasoning | ||
| 3 Developing | – Identifies difficult aspect | “This student did very well but got the answer wrong because they did not interpret the splitting pattern correctly. They understood that there are 2 degrees of unsaturation and two methyl [groups] but did not understand they the ketones must be placed to generate the two singlet peaks.” |
| – Provides reasoning relating to one PCK component: | ||
| Learner's prior knowledge | ||
| Conceptual teaching strategies | ||
| Representations (examples or models) | ||
| Curricular saliency | ||
| 4 Exemplary | – Identifies difficult aspect | “The student most likely had trouble recognizing their symmetry mistake because of the…ketone groups pointing different ways. They also probably have trouble visualizing where hydrogen [atoms] go so I would encourage them to allow them in. They totally missed the singlets so it is possible they do not understand what coupling means so that should also be explained.” |
| – Provides reasoning relating to two or more | ||
| PCK components | ||
| Teaching strategies score | Participant response | |
|---|---|---|
| 1 Limited | – Provides problem-solving method | “To help, I would help them figure out which protons are interacting with each other. I would then have them figure out what the ppm shift means for the amount of deshielding present for each proton. From there, I would help them piece the molecule together based on these observations.” |
| – Does not relate method to problem | ||
| 2 Basic | – Provides problem-solving method | “I would tell them to adjust the placement of the oxygen (carbonyl) to achieve different variants of splitting patterns and observe if the ppm ranges make sense based on the functional groups provided.” |
| – Relates method to problem | ||
| 3 Developing | – Uses interactive teaching, e.g. questioning to probe or promote students’ understanding | “Since they are so close to the correct answer, I would ask them how they could modify this molecule to generate singlet peaks. By having them identify the protons that cause the triplet and quartet peaks, they could hopefully see that half the compound is correct. By guess and check they should be able to figure out where to position the ketone correctly.” |
| or | ||
| – Uses illustrations or models during explanations | ||
| 4 Exemplary | – Recognizes learners’ prior knowledge | “The student knew that the structure contained an ethyl group, but they should have realized that the structure they drew cannot account for the two singlets. In this case, I would suggest a guess and check approach. Just start drawing structures, keeping the ethyl group but moving the other carbons around to get different connectivity. Keep changing the structure until all the NMR features are accounted for. I find that to be generally a good approach for me, as well as the students I have taught.” |
| What makes a topic difficult score | Participant response | |
|---|---|---|
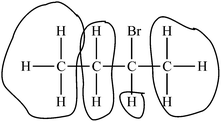
| 1 Limited | – Identifies difficult aspect | “Bromine creates diastereotopic protons.” |
| – Provides no reasoning | ||
| 2 Basic | – Identifies difficult aspect | “They are right for the most part except for the starred [hydrogen atoms]. These are diastereotopic and will show up differently on an NMR spectrum.” |
| – Provides broad and generic reasoning | ||
| 3 Developing | – Identifies difficult aspect | “The greatest difficulty here is the appearance of pseudo-symmetrical qualities in the molecule. The student does not fully understand that the Bromine disrupts the symmetry as it generates a stereocenter.” |
| – Provides reasoning relating to one PCK component: | ||
| Learner's prior knowledge | ||
| Conceptual teaching strategies | ||
| Representations (examples or models) | ||
| Curricular saliency | ||
| 4 Exemplary | – Identifies difficult aspect | No examples. |
| – Provides reasoning relating to two or more | ||
| PCK components | ||
| Teaching strategies score | Participant response | |
|---|---|---|
| 1 Limited | – Provides problem-solving method | “I would encourage students to find centers of chirality first (in these types of questions) and then determine whether this might affect the chemical environments of protons that are near (1 carbon away from) the stereocenter.” |
| – Does not relate method to problem | ||
| 2 Basic | – Provides problem-solving method | “You are correct in saying that the –CH3 methyl groups are different from one another. However, the –CH2 group is not only 1 group of hydrogens. Those two hydrogens are distinct from the other. The explanation for this relies on the deuterium test. If we deuterated one of those hydrogen [atoms], then a stereocenter is formed; however, there is another stereocenter in the molecule. Therefore, we just formed a diastereomer. If you deuterated the other hydrogen, then you form the opposite diastereomer. Seeing as diastereomers form with the deuterium test, these two hydrogens are different and will be individual ‘groups.’” (No illustrations included in response) |
| – Relates method to problem | ||
| 3 Developing | – Uses interactive teaching, e.g. questioning to probe or promote students’ understanding | [Participant included drawing of two Newman projections of 2-bromobutane with deuterium individually substituted for each diastereotopic hydrogen atom] “Ask the student if any chiral centers are present. ‘How do we check if protons adjacent to chiral centers are equivalent?’ Have the student draw both or draw one myself (student draw the other) of structure-projections above. Explain HA is between Br + H while HB is between Br + CH3.” |
| or | ||
| – Uses illustrations or models during explanations | ||
| 4 Exemplary | – Recognizes learners’ prior knowledge | “When I was a [TA for Organic Chemistry II lab], we taught students that chirality would make two or more protons on an adjacent carbon or adjacent carbons inequivalent. I would also stress in class that with really good NMRs, you may be able to see proton differences on further carbons but we will only grade nearest neighbors as different. I might use a model kit to explain that a certain “face” of the molecule will have protons interacting with the bromine or proton on the chiral center similar to how we explain rings to students.” |
Table 12: Responses to second tier of PCK questions
| PCK question | Prior teaching experience in organic chemistry course | Prior teaching experience in another chemistry course | Experience as a student | Research experience with NMR spectroscopy | Observing an experienced teacher | Other | Mean logit score |
|---|---|---|---|---|---|---|---|
| PCKQ1 | 16 | 0 | 14 | 10 | 5 | 2 | −1.25 |
| PCKQ2 | 15 | 1 | 16 | 10 | 5 | 0 | −0.41 |
| PCKQ3 | 15 | 1 | 14 | 11 | 6 | 0 | 0.28 |
| PCKQ4 | 15 | 1 | 13 | 6 | 6 | 0 | 1.39 |
Appendix 2: CK component of questionnaire
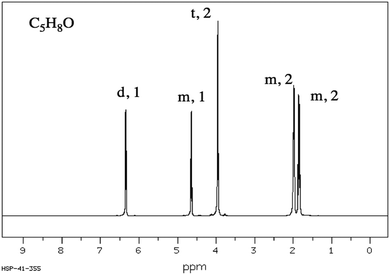
CKQ1. Determine the molecular structure corresponding to the given molecular formula and 1H NMR spectrum.CKQ2. Determine the molecular structure corresponding to the given molecular formula and 1H NMR spectrum.
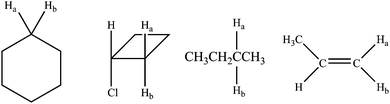
CKQ3. In which molecules are Ha and Hb equivalent?
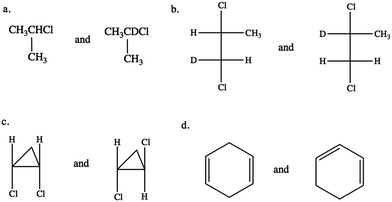
CKQ4. Circle all molecule pairs that are distinguishable using 1H NMR spectroscopy:
CKQ5. How many 1H NMR signals are produced by the following compound?
Appendix 3: PCK component of questionnaire
PCKQ1. What aspects of NMR spectral interpretation are most challenging for introductory organic chemistry students? Please be as specific as possible and provide a response that is complete and concise.PCKQ2. A student brings the 1H NMR spectrum and chemical shift table depicted below to your office hours. The student says that s/he is having trouble figuring out the corresponding molecular structure.
(a) Identify and describe particular features (if any) in the 1H NMR spectrum that may be creating difficulty for the student.
(b) As though you are talking with the student, explain how s/he should go about interpreting the spectrum below.
Please provide a sufficiently detailed response that completely captures your thinking. Extra space is provided on the next page.
Proton chemical shift table provided to Organic Chemistry II laboratory students for problem solving:
PCKQ3. You are grading problem #1 on a student's quiz (see below).
(a) For problem #1, was this student able to provide the correct answer? What (if any) features contributed to difficulty?
(b) If incorrect, how would you help this student correctly interpret this spectrum?
Please provide a sufficiently detailed response that completely captures your thinking. Extra space is provided on the next page.
Problem #1
Determine the molecular structure corresponding to the given molecular formula and 1H NMR spectrum.
Proton chemical shift table provided to Organic Chemistry II laboratory students for problem solving:
PCKQ4. You are now grading problem #2 on a student's quiz (see below).
(a) For problem #2, was this student able to provide the correct answer? What (if any) features contributed to difficulty?
(b) If incorrect, how would you help this student determine the correct answer?
Please provide a sufficiently detailed response that completely captures your thinking. Extra space is provided on the next page.
Problem #2
Circle all groups of equivalent hydrogen atoms.
References
- Alexander P., Schallert D. and Hare V., (1991), Coming to terms: how researchers in learning and literacy talk about knowledge, Rev. Educ. Res., 61(3), 315–343 DOI:10.3102/00346543061003315.
- American Chemical Society, (2015), Undergraduate professional education in chemistry: ACS guidelines and evaluation procedures for bachelor's degree programs, Washington, DC: American Chemical Society.
- Angawi R. F., (2014), Using a problem solving-cooperative learning approach to improve students’ skills for interpreting 1H NMR spectra of unknown compounds in an organic spectroscopy course, J. Chem. Educ., 91(6), 823–829.
- Ausubel D. P., Novak J. D. and Hanesian H., (1978), Educational psychology: a cognitive view, Chicago, IL: Holt, Rinehart and Winston.
- Bodner M. G. and Domin D. S., (2000), Mental Models: The Role of Representations in Problem Solving in Chemistry, Univ. Chem. Educ., 4(1), 24–30.
- Bond T. G. and Fox C. M., (2007), Applying the Rasch Model: Fundamental Measurement in the Human Sciences, New York: Routledge.
- Bruice P. Y., (2011), Organic Chemistry, 6th edn, Upper Saddle River: Prentice Hall.
- Carlson R. E., (1990), Assessing teachers’ pedagogical content knowledge: item development issues, J. Pers. Eval. Educ., 4, 157–173.
- Cartrette D. P. and Bodner G. M., (2010), Non-Mathematical Problem Solving in Organic Chemistry, J. Res. Sci. Teach., 47, 643–660.
- Charalambous C. Y., Hill H. C. and Ball D. L., (2011), Prospective teachers’ learning to provide instructional explanations: how does it look and what might it take? J. Math. Teach. Educ., 14(6), 441–463.
- ChemDraw Professional 16.0, (2017), PerkinElmer Informatics.
- Chin C., (2007), Teacher questioning in science classrooms: approaches that stimulate productive thinking, J. Res. Sci. Teach., 44, 815–843.
- Cochran K. F., (1993), Pedagogical content knowing: an integrative model for teacher preparation, J. Teach. Educ., 44, 263–272.
- Collins D., (2003), Pretesting survey instruments: an overview of cognitive methods, Qual. Life Res., 12(3), 229–238.
- Cronbach L. J. and Meehl P. E., (1955), Construct validity in psychological tests, Psychol. Bull., 52(4), 281–302.
- Dragisich V., Keller V. and Zhao M., (2016), An intensive training program for effective teaching assistants in chemistry, J. Chem. Educ., 93(7), 1204–1210.
- Gerothanassis I. P., Troganis A., Exarchou V. and Barbarossou K., (2002), Nuclear Magnetic Resonance (NMR) Spectroscopy: Basic Principles and Phenomena, and their Applications to Chemistry, Biology, and Medicine, Chem. Educ. Res. Pract. Eur., 3(2), 229–252.
- Gess-Newsome J., (2015), A model of teacher professional knowledge and skill including PCK: results of the thinking from the PCK summit, in Berry A., Friedrichsen P. and Loughran J. (ed.), Re-examining Pedagogical Content Knowledge in Science Education, New York: Routledge, pp. 28–42.
- Hale L., Lutter J. C. and Shultz G. V., (2016), The development of a tool for measuring graduate students’ topic specific pedagogical content knowledge of thin layer chromatography, Chem. Educ. Res. Pract., 17, 700–710.
- Haynes S. N., Richard D. C. S. and Kubany E. S., (1995), Content validity in psychological assessment: A functional approach to concepts and methods, Psychol. Assess., 7(3), 238–247.
- Hill H. C., Rowan B. and Ball D. L., (2005), Effects of Teachers’ Mathematical Knowledge for Teaching on Student Achievement, Am. Educ. Res. J., 42(2), 371–406 DOI:10.3102/00028312042002371.
- Hill H. C., Ball D. L. and Schilling S. G., (2008), Unpacking Pedagogical Content Knowledge: Conceptualizing and Measuring Teachers’ Topic-Specific Knowledge of Students, J. Res. Math. Educ., 39(4), 372–400.
- Jüttner M., Boone W., Park S. and Neuhaus B. J., (2013), Development and use of a test instrument to measure biology teachers’ content knowledge (CK) and pedagogical content knowledge (PCK), Educ. Assess. Eval. Acc., 25, 45–67.
- Kelly P., (2006), What is teacher learning? A socio-cultural perspective, Oxford Rev. Educ., 32(4), 505–519 DOI:10.1080/03054980600884227.
- Kind V., (2009), Pedagogical content knowledge in science education: perspectives and potential for progress, Stud. Sci. Educ., 45, 169–204.
- Landis J. R. and Koch G. G., (1977), The Measurement of Observer Agreement for Categorical Data, Biometrics, 33(1), 159–174.
- Linacre J. M., (2004), Test Validity and Rasch Measurement: Construct, Content, etc. Rasch Meas. Trans., 18(1), 970–971.
- Linacre J. M., (2017a), A user's guide to Winsteps/Ministep: Rasch-model computer programs, retrieved from http://www.winsteps.com/a/winsteps.pdf.
- Linacre J. M., (2017b), A user's guide to Facets: Rasch-model computer programs, retrieved from http://www.winsteps.com/manuals.htm.
- Lorigan G. A., Minto R. E. and Zhang W., (2001), Teaching the Fundamentals of Pulsed NMR Spectroscopy in an Undergraduate Physical Chemistry Laboratory, J. Chem. Educ., 78(7), 956–958.
- Malcolm S., (2015), The design and validation of an instrument to measure topic-specific pedagogical content knowledge in stoichiometry of physical sciences teachers, Master of Science Thesis, University of the Witwatersrand DOI:10.13140/RG.2.1.5174.2161.
- Mavhunga E., (2014), Improving PCK and CK in pre-service chemistry teachers, in Venkat H., Rollnick M., Askew M. and Loughran R. (ed.), Exploring Mathematics and Science Teachers' Knowledge: Windows into teacher thinking, Abingdon: Routledge, pp. 31–48.
- Mavhunga E., (2016), Transfer of the pedagogical transformation competence across chemistry topics, Chem. Educ. Res. Pract., 17(4), 1081–1097.
- Mavhunga E. and Rollnick M., (2013), Improving PCK of chemical equilibrium in pre-service teachers, Afr. J. Res. Math., Sci. Technol. Educ., 17, 113–125.
- Park S. and Chen Y. C., (2012), Mapping out the integration of the components of pedagogical content knowledge (PCK): examples from high school biology classrooms, J. Res. Sci. Teach., 49(7), 922–941 DOI:10.1002/tea.21022.
- Park S., Jang J., Chen Y. and Jung J., (2011), Is Pedagogical Content Knowledge (PCK) Necessary for Reformed Science Teaching?: Evidence from an Empirical Study, Res. Sci. Educ., 41, 245–260 DOI:10.1007/s11165-009-916.
- Park S. and Oliver J. S., (2008), Revisiting the conceptualization of pedagogical content knowledge (PCK): PCK as a conceptual tool to understand teachers as professionals, Res. Sci. Educ., 38, 261–284.
- Pitjeng P., (2014), Novice unqualified science teachers’ topic-specific pedagogical content knowledge and their beliefs about teaching, in Venkat H., Rollnick M., Askew M. and Loughran R. (ed.), Exploring Mathematics and Science Teachers’ Knowledge: Windows into teacher thinking, Abingdon: Routledge, pp. 65–83.
- Raker J. R., Reisner B. A., Smith S. R., Stewart J. L., Crane J. L., Pesterfield L. and Sobel S. G., (2015), Foundation Coursework in Undergraduate Inorganic Chemistry: Results from a National Survey of Inorganic Chemistry Faculty, J. Chem. Educ., 92(6), 973–979.
- Rollnick M., Bennett J., Rhemtula M., Dharsey N. and Ndlovu T., (2008), The place of subject matter knowledge in pedagogical content knowledge: a case study of South African teachers teaching the amount of substance and chemical equilibrium, Int. J. Sci. Educ., 30(10), 1365–1387.
- Rollnick M., Davidowitz B. and Potgieter M., (2017), Is Topic-Specific PCK Unique to Teachers? in Hahl K., Juuti, K. Lampiselkä J., Uitto A. and Lavonen J. (ed.), Cognitive and Affective Aspects in Science Education Research: Selected Papers from the ESERA 2015 Conference, Cham: Springer International Publishing, pp. 69–85.
- Rollnick M. and Mavhunga E., (2015), The PCK Summit and its Effect on Work in South Africa, in Berry A., Friedrichsen P. and Loughran J. (ed.), Re-examining Pedagogical Content Knowledge in Science Education, New York: Routledge, pp. 135–146.
- Rowan B., Schilling S. G., Ball D. L. and Miller R., (2001), Measuring teachers' pedagogical content knowledge in surveys: an exploratory study, State College, PA: Consortium for Policy Research in Education, Study of Instructional Improvement, http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.591.1047&rep=rep1&type=pdf.
- Schmelzing S., Van Driel J. H., Jüttner M. Brandenbusch S., Sandmann A. and Neuhaus B. J., (2013), Development, Evaluation, and Validation of a Paper-and-Pencil Test for Measuring Two Components of Biology Teachers’ Pedagogical Content Knowledge Concerning the “Cardiovascular System”, Int. J. Sci. Math. Educ., 11(6), 1369–1390 DOI:10.1007/s10763-012-9384-6.
- SDBS, (1997), Spectral database for organic compounds, National institute of advanced Industrial science and technology, http://sdbs.db.aist.go.jp/sdbs/cgibin/direct_frame_disp.cgi?sdbsno=2119 and http://sdbs.db.aist.go.jp/sdbs/cgi-bin/direct_frame_disp.cgi?sdbsno=1039.
- Shulman L. S., (1986), Those who understand: knowledge growth in teaching, Educ. Res., 15, 4–14.
- Shulman L. S., (1987), Knowledge and teaching: foundations of the new reform, Harv. Educ. Rev., 57, 1–22.
- Shulman L. S., (2015), PCK: its genesis and exodus, in Berry A., Friedrichsen P. and Loughran J. (ed.), Re-examining Pedagogical Content Knowledge in Science Education, New York: Routledge, pp. 3–13.
- Simpson A. J., Mitchell P. J., Masoom H., Liaghati Mobarhan Y., Adamo A. and Dicks A. P., (2015), An oil spill in a tube: an accessible approach for teaching environmental NMR spectroscopy, J. Chem. Educ.92(4), 693–697.
- Smith P. S. and Banilower E. R., (2015), Assessing PCK: a new application of the uncertainty principle, in Berry A., Friedrichsen P. and Loughran J. (ed.), Re-examining Pedagogical Content Knowledge in Science Education, New York: Routledge, pp. 88–103.
- Topczewski A. M., Tang H., Kendhammer L. K. and Pienta N. J., (2017), NMR spectra through the eyes of a student: eye tracking applied to NMR items, J. Chem. Educ., 94(1), 29–37.
- Veal W. R. and MaKinster J. G., (2001), Pedagogical content knowledge taxonomies, http://unr.edu/homepage/crowther/ejse/vealmak.html.
- Winschel G. A., Everett R. K., Coppola B. P., Shultz G. V. and Lonn, S., (2015), Using Jigsaw-Style Spectroscopy Problem-Solving to Elucidate Molecular Structure through Online Cooperative Learning, J. Chem. Educ.92(7), 1188–1193.
- Wright N. T., (2016), Heteronuclear Multidimensional Protein NMR in a Teaching Laboratory, J. Chem. Educ.93(2), 287–291.
| This journal is © The Royal Society of Chemistry 2018 |