Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Novel pathway to produce high molecular weight kraft lignin–acrylic acid polymers in acidic suspension systems†
Fangong Kongab,
Shoujuan Wang*ab,
Weijue Gaob and
Pedram Fatehi *b
*b
aKey Laboratory of Pulp and Paper Science and Technology of Ministry of Education, China, Qilu University of Technology, Jinan, 250353, China
bChemical Engineering Department, Lakehead University, Thunder Bay, ON P7B 5E1, Canada. E-mail: pfatehi@lakeheadu.ca; nancy5921@163.com; Tel: +1-807-343-8697
First published on 29th March 2018
Abstract
Kraft lignin (KL) produced in kraft pulping process has a low molecular weight and solubility, which limits its application in industry. For the first time, KL was polymerized with acrylic acid (AA) in an acidic aqueous suspension system to produce a water soluble lignin–AA polymer with a high molecular weight in this work. The polymerization reaction was carried out using K2S2O8 as an initiator, and the influence of reaction conditions on the carboxylate group content and molecular weight of resultant lignin polymers was systematically investigated. The mechanism of polymerization of KL and AA was discussed fundamentally. The resulting lignin–AA polymer was characterized by Fourier Transform Infrared spectrophotometry (FTIR), proton nuclear magnetic resonance (1H-NMR) and elemental analyses. The results showed that the phenolic hydroxyl group (Ph-OH) content of KL promoted the polymerization under an acidic environment. Under the conditions of 1.5 wt% of initiator, 3.5 of pH, 10.0 of AA/lignin molar ratio, 0.15 mol L−1 of lignin concentration, 3 h and 80 °C, the carboxylate group content and the molecular weight of the polymer were 7.37 mmol g−1 and 7.4 × 105 g mol−1, respectively. The lignin–AA polymer was water soluble at a 10 g L−1 concentration and a pH higher than 4.5. Furthermore, the flocculation performance of lignin–AA polymer in an aluminium oxide suspension was evaluated. Compared with polyAA, the lignin–AA polymer was a more efficient flocculant for aluminium oxide suspension, which shows its potential to be used as a green flocculant in industry.
Introduction
Lignin is a natural biomacromolecule, found in wood and vascular plants. Over 60 million tons of lignin is produced in the pulp and paper industry annually in the world.1 Among technical lignins produced, kraft lignin is the most dominant one, but is mainly incinerated as a low cost fuel in the pulping industry leading to the waste of resources and growing environmental problems.2,3 With the depletion of fossil fuel and the improvement of environmental awareness, greater endeavors have been made on developing lignin-based materials. However, kraft lignin has not yet been utilized effectively.4,5Lignin is a highly stable and complex compound with a three-dimensional aromatic structure formed from three phenylpropanoid monomer units of guaiacyl, syringyl and p-hydroxyphenyl, connected by ether and carbon–carbon bond in an irregular form.6 Various modification techniques were carried out in the past to produce new lignin-based products for beneficial purposes.7 One of these modifications is the polymerization of lignin with functional monomers, which can increase both the molecular weight and number of functional groups on lignin structure. Chen et al.8 produced a polymer by reacting lignosulfonate with 1-ethenylbenzene to enhance thermal stability and molecular weight of the polymer. The polymerization of lignosulfonate with vinyl monomers, i.e., acrylic acid, acrylonitrile, and methyl methacrylate, was also studied in aqueous or organic solvents in the past via chemical radical starters9–12 or chemo-enzymatic starters,13 in order to produce high molecular weight products with high hydrophilic or hydrophobic properties. Meister et al.14,15 synthesized acrylamide with kraft lignin in dioxane solution and used it as a drilling mud additive. In another report, 1-phenylethylene–kraft lignin polymer was produced in dimethyl sulfoxide solution and used as an oil recovery agent. However, organic solvents, such as dioxane and dimethyl sulfoxide, were generally used for facilitating the homogeneous polymerization of kraft lignin and other monomers. However, these solvents are usually toxic, expensive and may need a complex recovery process after use, which hampers their practical application in industry.
In the past, the polymerization of lignin in aqueous solutions was assessed. Ibrahim et al.16 polymerized soda lignin with 2-acrylamido-2-methylpropane in 1 wt% NaOH solution, and the product, soda lignin polymer with a molecular weight of 2.6 × 106 g mol−1, was used as a drilling mud additive. Fang et al.17 also produced a corn-stalk lignin–acrylamide polymer in NaOH solution and used it as an adsorbent for dye removal from wastewater. However, there is no report about the polymerization of kraft lignin and acrylic acid in aqueous acidic suspensions, which is studied for the first time in this work.
As proven in the literature, the complex and heterogeneous structure of lignin played important roles in its polymerization.10,12,17–19 However, there are contradictory reports on the role of phenolic group in the polymerization of lignin: (1) the phenolic group acts as an inhibitor owing to the quinoid structure produced in the polymerization, which was observed in the polymerization of styrene with lignosulfonate18 or with hydrochloric softwood lignin;19 (2) the phenolic group acts as an active centre for the polymerization. It was observed that the conversion rates of acrylic monomers, i.e., AA, acrylonitrile and methyl methacrylate, were significantly accelerated in the presence of lignosulfonate.4,11 Therefore, the role of phenolic group on the polymerization of KL and AA is still not clear, but is crucial for understanding the polymerization of KL and AA from academic and industrial points of views. In the polymerization reaction, the aliphatic hydroxyl groups of KL might react with carboxylate group of poly acrylic acid (PAA) formed during the lignin–AA polymerization reaction through esterification, which would also graft PAA onto lignin.20 It is not clear if the esterification reaction would happen in an acidic heterogeneous condition, which is the second objective of this study.
In the work presented herein, the polymerization of kraft lignin with acrylic acid in an acidic aqueous solution was conducted using K2S2O8 as an initiator. The main aim of this study was to generate water soluble lignin–AA polymer with a high molecular weight, which will facilitate its application as a flocculant in wastewater systems. In addition, the influence of phenolic group on the polymerization efficiency was identified. This study also intended to report how the functional groups and molecular weight of softwood kraft lignin will be affected by this polymerization. The properties of the lignin–AA polymer were determined using a light laser scattering technique, and the flocculation performance of the resulting lignin–AA polymer for an aluminium oxide suspension, as a model suspension system for representing wastes of the mining industry, was evaluated by a photometric dispersion analyzer.
Experimental
Materials
Softwood kraft lignin sample with a molecular weight (Mw) of 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 890 g mol−1 was received from FPInnovations' pilot plant facilities in Thunder Bay, ON. The kraft lignin was produced via LignoForce™ technology.20 Polydiallyldimethyl-ammonium chloride (PDADMAC, 100
890 g mol−1 was received from FPInnovations' pilot plant facilities in Thunder Bay, ON. The kraft lignin was produced via LignoForce™ technology.20 Polydiallyldimethyl-ammonium chloride (PDADMAC, 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–200
000–200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1), acrylic acid (AA), potassium persulfate (K2S2O8) (analytical grades), sodium hydroxide (97%, reagent grade), hydrochloric acid (37%, reagent grade), potassium hydroxide (8 mol L−1 solution), potassium permanganate (analytical grades), ferrous ammonium sulfate (analytical grades), 0.1 mol L−1 hydrochloric acid, D2O, trimethylsilyl propanoic acid (TMSP), dimethyl sulphate and para-hydroxybenzoic acid (analytical grades) were obtained from Sigma-Aldrich Company, and used as received. Dialysis membrane (cut off of 1000 g mol−1) was obtained from Spectrum Labs.
000 g mol−1), acrylic acid (AA), potassium persulfate (K2S2O8) (analytical grades), sodium hydroxide (97%, reagent grade), hydrochloric acid (37%, reagent grade), potassium hydroxide (8 mol L−1 solution), potassium permanganate (analytical grades), ferrous ammonium sulfate (analytical grades), 0.1 mol L−1 hydrochloric acid, D2O, trimethylsilyl propanoic acid (TMSP), dimethyl sulphate and para-hydroxybenzoic acid (analytical grades) were obtained from Sigma-Aldrich Company, and used as received. Dialysis membrane (cut off of 1000 g mol−1) was obtained from Spectrum Labs.
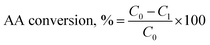 | (1) |
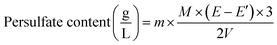 | (2) |
| Sample | KL | 1 | 2 | 3 |
| Dimethyl sulphate/phenolic group of KL, mol mol−1 | 0 | 0.25 | 0.50 | 1.0 |
| Ph-OH group, mmol g−1 lignin | 1.73 | 1.41 | 1.02 | 0.70 |
| Carboxylate group,mmol g−1 lignin | 0.37 | 0.38 | 0.36 | 0.36 |
| Sample | Lignin concentration, wt% | H2O2, wt%, based on lignin | Time, h | Temperature, °C | Carboxylate group, mmol g−1 | Ph-OH, mmol g−1 |
|---|---|---|---|---|---|---|
| 1 | 5 | 18 | 1 | 90 | 1.65 | 0.74 |
| 2 | 5 | 8 | 1 | 80 | 1.06 | 0.95 |
| 3 | 5 | 4 | 1 | 80 | 0.65 | 1.23 |
| 4 | 5 | 4 | 0.5 | 80 | 0.43 | 1.39 |
| KL | — | — | — | — | 0.37 | 1.73 |
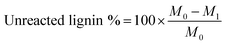 | (3) |
 respectively). The corresponding three endpoints in the titration curve of blank sample were specified as V1, V2 and V3, respectively. The carboxylate group and Ph-OH contents of samples were calculated according to eqn (4) and (5).29 The reported data in this paper is the average of three repetitions.
respectively). The corresponding three endpoints in the titration curve of blank sample were specified as V1, V2 and V3, respectively. The carboxylate group and Ph-OH contents of samples were calculated according to eqn (4) and (5).29 The reported data in this paper is the average of three repetitions.
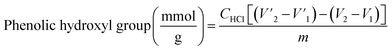 | (4) |
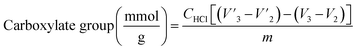 | (5) |
About 100 mg of air dried KL sample was initially suspended in 4.0 mL of acetic anhydride/pyridine 1/1 (v/v) solution by stirring for 30 min at 300 rpm and 25 °C; and then the solution containing KL was kept in a dark place at 25 °C for 24 h to acetylate KL. The resulting solution was then poured in an excess amount (50 mL) of ice water and centrifuged/washed 3 times. Afterwards, the solvent was removed from the samples using a freeze dryer. The acetylated KL was dissolved in 10 mL of tetrahydrofuran (THF) by stirring at 300 rpm for 30 min at room temperature, and then filtered with a PTFE filter having a diameter of 13 mm and pore size of 0.2 μm. The filtered samples were used for a molecular weight analysis. For lignin–AA polymer analysis, about 50 mg of air dried polymer samples was dissolved in 10 mL of 0.1 mol L−1 NaNO3 solution and filtered with a 0.2 μm nylon filter (13 mm diameter). The filtered solutions were used for molecular weight analysis.
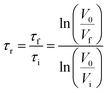 | (6) |
Results and discussions
Reaction mechanism of polymerization of KL and AA
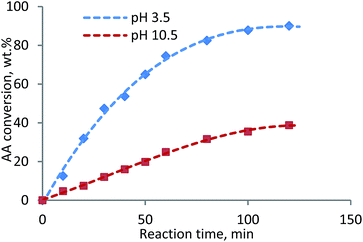
The final KL–AA polymer from acidic and alkaline systems has very different charge density (1.86 meq g−1 under alkaline vs. 7.22 meq g−1 under acidic condition) and molecular weight (0.46 × 105 g mol−1 under alkaline vs. 7.4 × 105 g mol−1 under acidic condition). The AA conversion of AA homopolymerization system at pH 3.5 and 10.5 was measured and presented in Fig. 1. The AA conversion of reaction at pH 3.5 reached 90.1% in 2 h, however, it was only 38.8% at pH 10.5. Also, the molecular weights of PAAs from the reaction conducted at pH 3.5 and pH 10.5 were determined to be 4.26 × 105 g mol−1 and 0.83 × 105 g mol−1, respectively. This phenomenon was also observed in another work.35 One can conclude that the higher charge density and molecular weight of KL–AA polymer, prepared under acidic condition (pH 3.5), could be due to the fact that lignin accelerated the radical formation and thus more AA conversion.
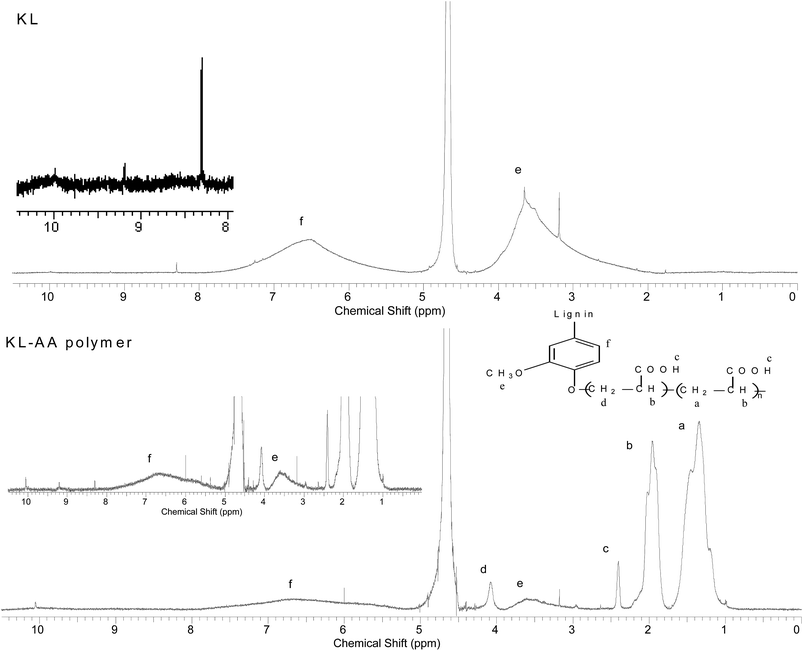
In this study, the polymerization of KL and AA was carried out in an acidic aqueous suspension through heterogeneous reaction. To clarify if KL and PAA react via esterification, the PAA prepared in this study was used for investigating the reaction between KL and PAA with and without an initiator. The 1H-NMR analysis of the products of KL and PAA reaction, which is available in ESI (Fig. S3†), showed that no PAA was grafted onto KL; illustrating that (i) the esterification reaction between carboxylate groups of PAA and aliphatic hydroxyl groups of KL did not occur in the acidic system, and that (ii) the terminated PAA formed during the polymerization reaction of KL and AA cannot be reinitiated to form KL–AA polymers.
It was reported that the polymerization of styrene and hydrochloric acid lignin did not occur on aliphatic group of lignin molecules.19,40 In our previous work, we described that the polymerization did not occur on those groups in alkaline homogenous reaction of KL and AA.21 Due to the lowest bond dissociation energies of C6H5O–H (89.8 kcal mol−1), and C6H5CH2–H (90 kcal mol−1) among chemical groups in KL,41,42 the predominant lignin radicals, which are induced by the initiator radicals SO42−˙ through the homolytic rupture of a bond in KL during acidic polymerization reaction, are phenoxyl radicals from phenolic lignin units, and with smaller probability, benzylic radicals from non-phenolic lignin units. It was also reported in the literature42 that acetylated lignin models had significantly lower free radicals (benzylic radicals) than the untreated lignin samples. To clarify whether the polymerization of KL and AA occurred through the benzylic radicals in the acidic conditions, the acetylated KL was used to polymerize with AA. The final product was analyzed using 1H-NMR (Fig. S4 in ESI†) and showed that the characteristic peaks of PAA did not exist on the product of acetylated KL and AA; demonstrating that (i) the polymerization of AA onto KL through benzylic radicals was not detectable in acidic system, (ii) in the absence of phenolic hydroxyl group, the aromatic ring, methoxyl group and aliphatic portion of lignin molecules did not participate in the reaction, and (iii) in the absence of phenolic hydroxyl group in KL, the polymerization of AA and KL was not noticed in acidic conditions.
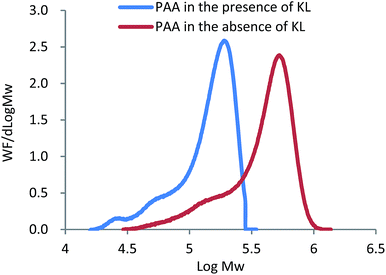
In addition to the lignin radicals formed by the initiator, the chain transfer reactions of KL and the growing PAA radicals may form lignin radicals. To testify if KL functions as a radical transfer in this system, the molecular weight of PAA formed in the absence and presence of KL was measured and shown in Fig. 2.
As presented in Fig. 2, the molecular weight of PAA in the presence of KL (111![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 g mol−1) was much lower than that in the absence of KL (426
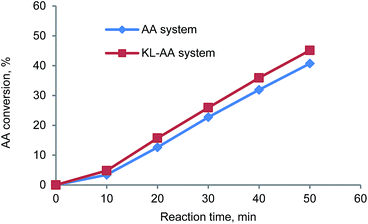
700 g mol−1) was much lower than that in the absence of KL (426![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 g mol−1), illustrating that (i) KL functioned as a chain transfer agent in this polymerization system, and (ii) the chain transfer reaction between KL and PAA chain radicals formed some lignin radicals for this polymerization. As it is well known, the chain transfer in polymerization system not only results in a reduced molecular weight of the polymer, but may also affect the polymerization rate, which depends on the reinitiation reaction rate between chain transfer radicals and monomers. In order to understand the effect of KL on the polymerization rate, the AA conversion in KL–AA polymerization system and AA homopolymerization system was determined and shown in Fig. 3. It is apparent that KL slightly increased the AA conversion, illustrating that the reinitiation reaction rate between lignin radicals and monomers is similar or slightly higher than that of propagation reaction of AA chain radicals, which is also consistent with the findings in the polymerization of lignosulfonate with AA in the literature.43 The reason for this behaviour can be ascribed to a greater decomposition of the initiator in the presence of KL (as discussed earlier).
300 g mol−1), illustrating that (i) KL functioned as a chain transfer agent in this polymerization system, and (ii) the chain transfer reaction between KL and PAA chain radicals formed some lignin radicals for this polymerization. As it is well known, the chain transfer in polymerization system not only results in a reduced molecular weight of the polymer, but may also affect the polymerization rate, which depends on the reinitiation reaction rate between chain transfer radicals and monomers. In order to understand the effect of KL on the polymerization rate, the AA conversion in KL–AA polymerization system and AA homopolymerization system was determined and shown in Fig. 3. It is apparent that KL slightly increased the AA conversion, illustrating that the reinitiation reaction rate between lignin radicals and monomers is similar or slightly higher than that of propagation reaction of AA chain radicals, which is also consistent with the findings in the polymerization of lignosulfonate with AA in the literature.43 The reason for this behaviour can be ascribed to a greater decomposition of the initiator in the presence of KL (as discussed earlier).
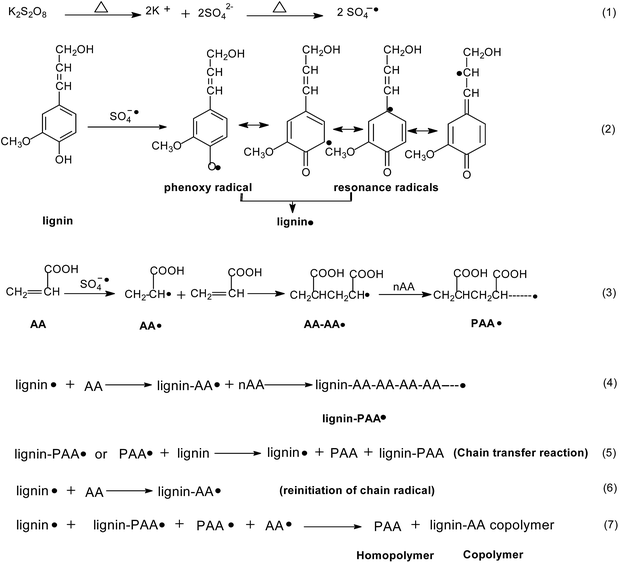
Based on the analysis above, the proposed reaction scheme of KL and AA polymerization is shown in Scheme 1. As softwood KL is known to be composed principally of coniferyl alcohol units,44 it was chosen to present KL in this scheme. In this polymerization reaction, the sulfate radicals can initially be formed by heat decomposition (reaction (1)), which then react with phenolic hydroxyl group of KL to generate phenoxy radicals and its resonance radicals (reaction (2)). The formation of the KL radicals will lead to more decomposition in reaction (1) and more sulfate radicals. Also, the sulfate radicals can initiate AA to form AA radicals (AA˙). The AA radicals then react with other AA monomers to form PAA chain radicals (reaction (3)).9,11 The KL radicals (lignin˙) react with monomer (AA) to form propagated lignin–AA chain radicals (reaction (4)). In addition, the PAA chain radicals and lignin–AA radicals in the system can react with KL to form KL radicals through a chain radical transfer reaction (reaction (5)), and the KL radicals then reinitiate AA to form lignin–AA chain radicals (reaction (6)). Finally, the propagated AA chain radicals and propagated lignin–AA chain radicals react with each other to produce PAA homopolymer and lignin–AA polymer through termination reaction as shown in reactions (7). As a result, the carboxylate groups are introduced onto KL and the molecular weight of KL is increased.
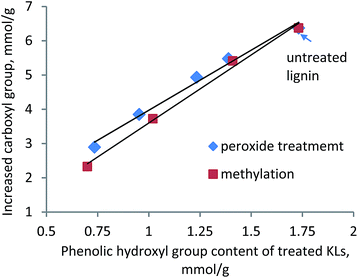
In another set of experiments, the impact of Ph-OH content of KL on the polymerization efficiency was determined via treating KL with hydrogen peroxide, which can reduce the Ph-OH content of lignin.45,46 The H2O2 treatment conditions and the Ph-OH content of the treated lignin are listed in Table 2. As seen in Table 2, by increasing the dosage of H2O2, the temperature and time of the treatment, the Ph-OH content of the resulting KLs decreased; whereas, the carboxylate content of the treated KLs increased, which was due to the oxidation of KL by H2O2.46,47 The treated and untreated KLs were polymerized with AA, and the carboxylate group of the resulting lignin–AA polymer was measured. Fig. 4 presents the impact of Ph-OH of KL on carboxylate content of KL–AA polymer. It can be seen that the Ph-OH content of lignin has a linear relationship with the increased carboxylate group of the final polymer, indicating that the OH group attached to the phenolic structure of KL is the reaction site for the polymerization.
To further assess the relationship between phenolic hydroxyl group of KL and increased carboxyl group of KL–AA polymer, the methylated lignins with different amounts of phenolic group were polymerized with AA. The increased carboxylate group had a linear relationship with the phenolic hydroxyl group of KL (Fig. 4), demonstrating the importance of phenolic hydroxyl group of KL on polymerization. It is also seen in this figure that, at a high amount of phenolic hydroxyl group on either peroxide-treated lignin or methylated lignin, the increased carboxylate groups for lignin–AA polymers are similar. However, with decreasing the amount of the phenolic hydroxyl group, the increased carboxylate group of the resulting polymer was more pronounced for peroxide-treated KL.
The reasons for this phenomenon might be (i) the more open structure of lignin after peroxide treatment compared with that after methylation, and (ii) the lower molecular weight and higher solubility of peroxide-treated KL compared with methylated lignin. In other words, the more open structure provides higher accessibility of AA to the reaction sites on KL. To clarify this, the acetylated peroxide-treated lignin was reacted with AA and the final product was analyzed using 1H-NMR (Fig. S5† in ESI†). The results showed that AA was not polymerized with the acetylated peroxide-treated lignin, illustrating the fact that there were no other reaction sites on the peroxide-treated lignin. Therefore, it can be concluded that the more significant increase in the carboxylate group of peroxide-treated lignin–AA polymer was attributed to the easier accessibility of AA to the phenolic hydroxyl group of KL, as the reaction site for the polymerization was still phenolic group of peroxide treated KL. This phenomenon was also observed by Phillips et al.19 in the polymerization of styrene and calcium lignosulfonate.
| Sample | AA/lignin molar ratio, n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Time, h | Initiator, wt% on lignin mass | Percentage of unreacted lignin, % |
|---|---|---|---|---|
| a Other reaction condition: temperature 80 °C, lignin concentration 0.15 mol L−1, pH 3.5. | ||||
| 1 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.5 | 1.5 | 1.07 |
| 2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2 | 1.5 | 1.13 |
| 3 | 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2 | 1.5 | 1.08 |
| 4 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2 | 1.5 | 0.61 |
| 5 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2 | 0 | 99.3 |
| 6 | 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2 | 1.5 | 98.8 |
| 7 | 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2 | 0 | 99.5 |
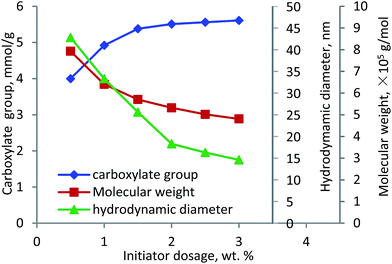
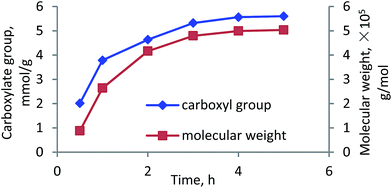
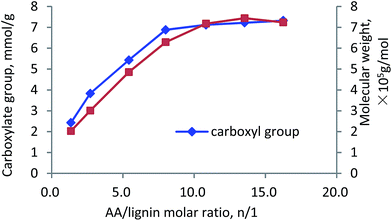
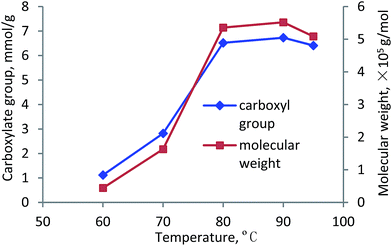
Effects of reaction conditions
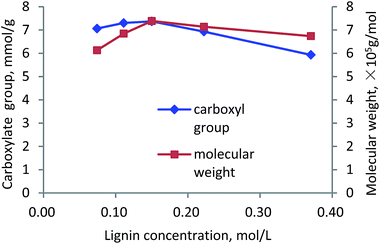 | ||
| Fig. 9 Carboxylate group content and molecular weight of lignin–AA polymer as a function of lignin concentration (pH, 3.5, initiator, 1.5 wt%, AA/lignin molar ratio, 10.0, 3 h, 80 °C). | ||
To investigate the relationship between carboxylate group and molecular weight of lignin–AA polymer, the data presented in previous figures is plotted in Fig. 10. As seen, the molecular weight of the polymer increased linearly with the increase in the carboxylate group content, indicating that the increase in the molecular weight of the polymer was mainly attributed to the PAA segment in the KL–AA polymer. The linear relationship between carboxylate group content and molecular weight is Y = 1.0618X − 0.9738, R2 = 0.9462, where Y is the molecular weight (×105 g mol−1) and X is the carboxylate group of KL–AA polymer (mmol g−1). This formula can be used to correlate the molecular weight of lignin–AA polymer with its carboxylate group content. In addition to the molecular weight, the Hy of lignin–AA polymers with different carboxylate group contents for the same samples were measured and presented in Fig. 10. The Hy of lignin–AA polymers did not show a linear relationship with the carboxylate group, illustrating that even though lignin–AA with a determined molecular weight can be generated, the formed lignin–AA polymer may have different molecular conformations (coiled/linear) in solutions if they were produced under different conditions. The higher Hy of samples with lower carboxylate group may imply that, when the amount of carboxylate group (and molecular weight) of polymer was low, the polymer was linear or more lignin was involved, but with high carboxylate group (and high MW) the polymer generated a coil shape conformation.
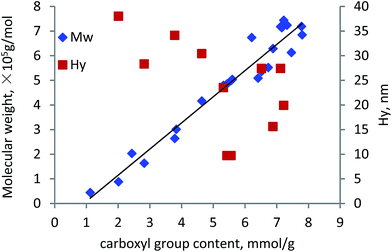 | ||
| Fig. 10 Relationship between carboxylate group content of lignin–AA polymer with its molecular weight (based on 1.5 wt% initiator). | ||
Based on the results shown in previous figures, the optimal conditions for producing lignin–AA polymer were 0.15 mol L−1 KL, AA/KL molar ratio 10.0, initiator 1.5 wt% (based on lignin mass), 80 °C and 3 h. Under these conditions, the carboxylate group and molecular weight of KL–AA polymer reached 7.37 mmol g−1 and 7.4 × 105 g mol−1, respectively. This lignin–AA polymer was selected for further analysis.
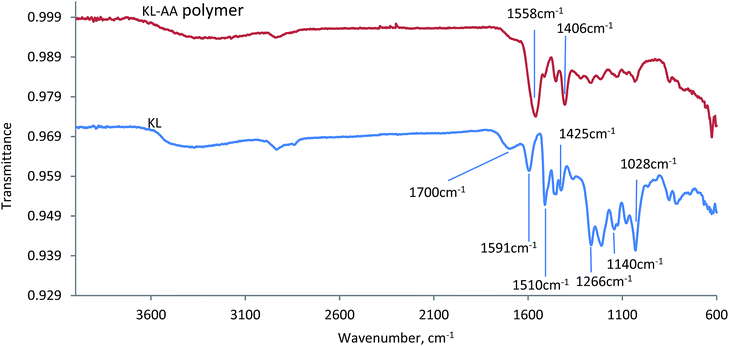
Characterization of lignin–AA polymer
| Sample | Carbon, wt% | Hydrogen, wt% | Oxygen, wt% | Formula | Carboxylate group, mmol g−1 | Mw, × 105 g mol−1 |
|---|---|---|---|---|---|---|
| KL | 62.6 | 5.69 | 27.04 | C9H9.60O2.85 | 0.37 | 0.17 |
| KL–AA | 45.3 | 3.92 | 31.69 | C9H9.34O4.72 | 7.37 | 7.4 |
Flocculation performance of KL–AA polymer for aluminium oxide suspension
Aluminum oxide is an industrially important oxide mineral. The flocculation of aluminum oxide particles is a key step for treatment of wastewater from mining industry.72 The flocculation characteristics of KL–AA polymer at different pHs were assessed in a 2.5 wt% aluminum oxide suspension, and the results are presented in Fig. 14. With an increase in the concentration, the flocculation efficiency of KL–AA was enhanced, but better results were obtained for KL–AA polymer at pH 6. As reported in the literature, flocculation can be promoted via charge neutralization, bridging, and hydrophobic/hydrophobic interaction.73,74 The reasons for better flocculation efficiency of KL–AA polymer at pH 6 are due to the fact that the (1) surface charge of aluminum oxide particles is positive at pH 6 (zeta potential, 12.4 mV), which can be neutralized by the negative charge of KL–AA polymer and (2) the KL segment of the KL–AA polymer offers the hydrophobic/hydrophobic interaction with aluminum oxide.73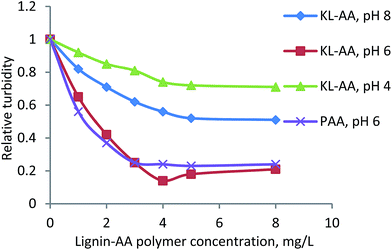 | ||
| Fig. 14 Flocculation performance of KL–AA polymer at different concentrations and pHs for aluminium oxide suspension. | ||
Furthermore, at a low concentration (e.g., 1 mg L−1 concentration), the flocculation efficiency of PAA is higher than that of KL–AA polymer. This is attributed to the amount of charges introduced to the particles. The carboxyl group content of PAA (i.e., 10.2 mmol g−1) is much higher than that of KL–AA polymer (i.e., 7.4 mmol g−1). At the same concentration, PAA offers more negative charges than KL–AA polymer to the surface of particles, providing a more neutralization degree to aluminum oxide particles and thus a higher flocculation efficiency. At 3 mg L−1 concentration for PAA and 4 mg L−1 concentration for KL–AA polymer, the amount of charges introduced to particles are similar, i.e., 29.5 mmol L−1 with KL–AA polymer vs. 30.6 mmol L−1 with PAA, inducing a similar turbidity for aluminum oxide suspensions. At higher dosages, a higher flocculation efficiency was achieved for KL–AA polymer than for PAA indicating that, in addition to the PAA segment in KL–AA polymer, the lignin segment in KL–AA polymer contributed to the flocculation (i.e., a more important role of KL component than PAA charge). Furthermore, the higher molecular weight (7.4 × 105 g mol−1) and higher Hy (25.2 nm) of KL–AA than those of PAA (4.26 × 105 g mol−1 and 7.2 nm) might have played roles in flocculation at the optimum dosage.
Conclusions
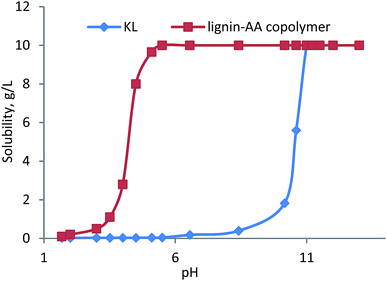
The polymerization mechanism of KL and AA under acidic conditions was comprehensively studied in this work. It was discovered that KL promoted the decomposition of the initiator under acidic conditions. Also, more PAA chain would be grafted on KL under acidic conditions than alkaline. The results suggested that the phenolic hydroxyl group content of KL had a significant influence on the AA polymerization. The optimal conditions for the polymerization were 0.15 mol L−1 KL, AA/KL ratio of 10.0 mol mol−1, 1.5 wt% initiator, 80 °C and 3 h. Under the optimized conditions, the carboxylate group content and the molecular weight of the KL–AA polymer were 7.22 meq g−1 and 7.4 × 105 g mol−1, respectively. The FTIR, H-NMR and elemental analyses confirmed the successful polymerization of KL and AA. Additionally, the resulting KL–AA polymer was water soluble at a pH higher than 4.5 and its maximum solubility was 100 g L−1. Compared with PAA, the KL–AA polymer was a more efficient flocculant for aluminum oxide suspensions at pH 6.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to acknowledge NSERC, Canada, Canada Foundation for Innovation (CFI), Ontario government and FPInnovations for supporting this research.Notes and references
- X. Zhang, M. B. Tu and M. G. Paice, BioEnergy Res., 2011, 4, 246–257 CrossRef.
- D. Stewart, Ind. Crops Prod., 2008, 27, 202–207 CrossRef CAS.
- F. H. Isikgor and C. R. Becer, Polym. Chem., 2015, 6, 4497–4559 RSC.
- D. Z. Ye, L. Jiang, C. Ma, M. H. Zhang and X. Zhang, Int. J. Biol. Macromol., 2014, 63, 43–48 CrossRef CAS PubMed.
- P. Fatehi, Curr. Org. Chem., 2013, 17, 1569 CrossRef CAS.
- C. Amen-Chen, H. Pakdel and C. Roy, Bioresour. Technol., 2001, 79, 277–299 CrossRef CAS PubMed.
- T. Aro and P. Fatehi, Chemsuschem, 2017, 10, 1861–1877 CrossRef CAS PubMed.
- M. J. Chen, D. W. Gunnells, D. J. Gardner, O. Milstein, R. Gersonde, H. J. Feine, A. Huttermann, R. Frund, H. D. Ludemann and J. J. Meister, Macromolecules, 1996, 29, 1389–1398 CrossRef CAS.
- D. Z. Ye, X. C. Jiang, C. Xia, L. Liu and X. Zhang, Carbohydr. Polym., 2012, 89, 876–882 CrossRef CAS PubMed.
- R. Chen and B. V. Kokta, ACS Symp. Ser., 1982, 187, 285–299 CrossRef CAS.
- R. Chen, B. V. Kokta and J. L. Valade, J. Appl. Polym. Sci., 1980, 25, 2211–2220 CrossRef CAS.
- P. P. Porto, E. O. S. Saliba, L. C. Goncalves, N. M. Rodriguez, I. Borges, A. L. C. C. Borges, J. A. S. Rodrigues and G. H. F. Ibrahim, Arq. Bras. Med. Vet. Zootec., 2006, 58, 99–107 CrossRef.
- C. Mai, A. Majcherczyk and A. Huttermann, Enzyme Microb. Technol., 2000, 27, 167–175 CrossRef CAS PubMed.
- J. J. Meister, D. R. Patil and H. Channell, J. Appl. Polym. Sci., 1984, 29, 3457–3477 CrossRef CAS.
- J. J. Meister, D. R. Patil and H. Channell, Abstr. Pap., Jt. Conf. - Chem. Inst. Can. Am. Chem. Soc., 1984, 187, 111 Search PubMed.
- M. N. M. Ibrahim, S. L. Lim, M. R. Ahmed-Haras and F. S. Fayyadh, Bioresources, 2014, 9, 1472–1487 CrossRef.
- R. Fang, X.-S. Cheng, J. Fu and Z.-B. Zheng, Natural Science, 2009, 1, 17 CrossRef CAS.
- R. Chen, B. V. Kokta and J. L. Valade, J. Appl. Polym. Sci., 1979, 24, 1609–1618 CrossRef CAS.
- R. Phillips, W. Brown and V. Stannett, J. Appl. Polym. Sci., 1971, 15, 2929–2940 CrossRef CAS.
- L. Kouisni, P. Holt-Hindle, K. Maki and M. Paleologou, J. Sci. Technol. For. Prod. Processes, 2012, 2, 6–10 Search PubMed.
- F. Kong, S. Wang, J. T. Price, M. K. Konduri and P. Fatehi, Green Chem., 2015, 17, 4356–4366 Search PubMed.
- M. V. Andes, Chim. Ind., 2006, 88, 88–95 Search PubMed.
- H. Sadeghifar, C. Z. Cui and D. S. Argyropoulos, Ind. Eng. Chem. Res., 2012, 51, 16713–16720 CrossRef CAS.
- R. Agnemo and G. Gellerstedt, Acta Chem. Scand., Ser. B, 1979, 33, 337–342 CrossRef.
- J. Gierer, Holzforschung, 1982, 36, 43–51 CrossRef CAS.
- J. Gierer, Holzforschung, 1982, 36, 55–64 CrossRef CAS.
- C. Schuerch, JACS, 1952, 74, 5061–5067 CrossRef CAS.
- J. R. Witono, J. H. Marsman, I. W. Noordergraaf, H. J. Heeres and L. P. Janssen, Carbohydr. Res., 2013, 370, 38–45 CrossRef CAS PubMed.
- M. Zhou, K. Huang, X. Qiu and D. Yang, CIESC J., 2012, 63, 258–265 CAS.
- M. F. Yan, D. J. Yang, Y. H. Deng, P. Chen, H. F. Zhou and X. Q. Qiu, Colloids Surf., A, 2010, 371, 50–58 CrossRef CAS.
- M. S. Jahan, D. N. Chowdhury, M. K. Islam and S. I. Moeiz, Bioresour. Technol., 2007, 98, 465–469 CrossRef CAS PubMed.
- R. E. Lappan, R. Pelton, I. McLennan, J. Patry and A. N. Hrymak, Ind. Eng. Chem. Res., 1997, 36, 1171–1175 CrossRef CAS.
- C. Ovenden and H. N. Xiao, Colloids Surf., A, 2002, 197, 225–234 CrossRef CAS.
- H. Xiao, Z. Liu and N. Wiseman, J. Colloid Interface Sci., 1999, 216, 409–417 CrossRef CAS PubMed.
- I. M. Kolthoff and I. K. Miller, JACS, 1951, 73, 3055–3059 CrossRef CAS.
- A. Romero, A. Santos, F. Vicente and C. Gonzalez, Chem. Eng. J., 2010, 162, 257–265 CrossRef CAS.
- D. Hunkeler, Macromolecules, 1991, 24, 2160–2171 CrossRef CAS.
- S. S. Panesar, S. Jacob, M. Misra and A. K. Mohanty, Ind. Crops Prod., 2013, 46, 191–196 CrossRef CAS.
- M. I. H. Mondal and M. M. U. Haque, J. Appl. Polym. Sci., 2007, 103, 2369–2375 CrossRef CAS.
- R. Phillips, W. Brown and V. Stannett, J. Appl. Polym. Sci., 1973, 17, 443–451 CrossRef.
- E. Baciocchi, M. Bietti and O. Lanzalunga, Acc. Chem. Res., 2000, 33, 243–251 CrossRef CAS PubMed.
- O. Lanzalunga and M. Bietti, J. Photochem. Photobiol., B, 2000, 56, 85–108 CrossRef CAS.
- D. Z. Ye, X. C. Jiang, C. Xia, L. Liu and X. Zhang, Carbohydr. Polym., 2012, 89, 876–882 CrossRef CAS PubMed.
- C. H. Ludwig and K. Sarkanen, Lignins: occurrence, formation, structure and reactions, Wiley-Interscience, 1971 Search PubMed.
- V. Kislenko and A. A. Berlin, Eur. Polym. J., 1996, 32, 1023–1029 CrossRef CAS.
- R. Agnemo and G. Gellerstedt, Acta Chem. Scand., Ser. B, 1979, 33, 337–342 CrossRef.
- D. Z. Ye, M. H. Zhang, L. L. Gan, Q. L. Li and X. Zhang, Int. J. Biol. Macromol., 2013, 60, 77–82 CrossRef CAS PubMed.
- A. K. Sarkar, N. Mandre, A. Panda and S. Pal, Carbohydr. Polym., 2013, 95, 753–759 CrossRef CAS PubMed.
- B. R. Nayak and R. P. Singh, Polym. Int., 2001, 50, 875–884 CrossRef CAS.
- R. L. Chen, B. V. Kokta, C. Daneault and J. L. Valade, J. Appl. Polym. Sci., 1986, 32, 4815–4826 CrossRef CAS.
- M. I. Khalil, K. M. Mostafa and A. Hebeish, Angew. Makromol. Chem., 1993, 213, 43–54 CrossRef CAS.
- J. R. Witono, I. W. Noordergraaf, H. J. Heeres and L. P. B. M. Janssen, Carbohydr. Polym., 2012, 90, 1522–1529 CrossRef CAS PubMed.
- A. Bayazeed, M. R. Elzairy and A. Hebeish, Starch-Stärke, 1989, 41, 233–236 CrossRef CAS.
- L. Petridis, R. Schulz and J. C. Smith, JACS, 2011, 133, 20277–20287 CrossRef CAS PubMed.
- A. Huttermann, A. Majcherczyk, A. Braun-Lullemann, C. Mai, M. Fastenrath, A. Kharazipour, J. Huttermann and A. H. Huttermann, Naturwissenschaften, 2000, 87, 539–541 CrossRef CAS PubMed.
- S. Kiatkamjornwong, W. Chomsaksakul and M. Sonsuk, Radiat. Phys. Chem., 2000, 59, 413–427 CrossRef CAS.
- Z. S. Fu, W. D. Liang, A. M. Yang and Z. G. Wang, J. Appl. Polym. Sci., 2002, 85, 896–899 CrossRef CAS.
- V. K. Thakur, A. S. Singha and M. K. Thakur, J. Polym. Environ., 2012, 20, 164–174 CrossRef CAS.
- L. Ayed, E. Khelifi, H. Ben Jannet, H. Miladi, A. Cheref, S. Achour and A. Bakhrouf, Chem. Eng. J., 2010, 165, 200–208 CrossRef CAS.
- M. N. M. Ibrahim, M. R. Ahmed-Haras, C. S. Sipaut, H. Y. Aboul-Enein and A. A. Mohamed, Carbohydr. Polym., 2010, 80, 1102–1110 CrossRef.
- K. K. Pandey, J. Appl. Polym. Sci., 1999, 71, 1969–1975 CrossRef CAS.
- A. Casas, M. V. Alonso, M. Oliet, E. Rojo and F. Rodriguez, J. Chem. Technol. Biotechnol., 2012, 87, 472–480 CrossRef CAS.
- N. E. El Mansouri and J. Salvado, Ind. Crops Prod., 2006, 24, 8–16 CrossRef CAS.
- J. L. Wang, Y. A. Zheng and A. Q. Wang, Adv. Mater. Res., 2010, 96, 227–232 CrossRef CAS.
- P. H. Mccluskey, R. L. Snyder and R. A. Condrate, J. Solid State Chem., 1989, 83, 332–339 CrossRef CAS.
- K. Lundquist, Acta Chem. Scand., Ser. B, 1981, 35, 497–501 CrossRef.
- M. Nagy, M. Kosa, H. Theliander and A. J. Ragauskas, Green Chem., 2010, 12, 31–34 RSC.
- L. H. Hu, H. Pan, Y. H. Zhou, C. Y. Hse, C. G. Liu, B. F. Zhang and B. Xu, J. Wood Chem. Technol., 2014, 34, 122–134 CrossRef CAS.
- Y. C. Kang, Z. Z. Chen, B. J. Wang and Y. Q. Yang, Ind. Crops Prod., 2014, 56, 105–112 CrossRef CAS.
- X. Y. Xiong, K. C. Tam and L. H. Gan, Macromolecules, 2003, 36, 9979–9985 CrossRef CAS.
- N. S. Nikouei and A. Lavasanifar, Acta Biomater., 2011, 7, 3708–3718 CrossRef PubMed.
- D. Feng, J. S. J. van Deventer and C. Aldrich, Sep. Purif. Technol., 2004, 40, 61–67 CrossRef CAS.
- T. W. Healy and V. K. La Mer, J. Colloid Sci., 1964, 19, 323–332 CrossRef CAS.
- S. Ghosh, G. Sen, U. Jha and S. Pal, Bioresour. Technol., 2010, 101, 9638–9644 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra12971h |
| This journal is © The Royal Society of Chemistry 2018 |