Photoredox-catalyzed cascade annulation of methyl(2-(phenylethynyl)phenyl)sulfanes and methyl(2-(phenylethynyl)phenyl)selanes with sulfonyl chlorides: synthesis of benzothiophenes and benzoselenophenes†
Abstract
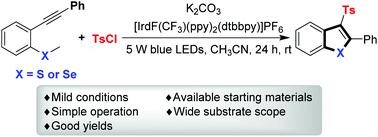
A photoredox-catalyzed cascade annulation of methyl(2-(phenylethynyl)phenyl)sulfanes and methyl(2-(phenylethynyl)phenyl)selanes with sulfonyl chlorides was developed. A variety of benzothiophenes and benzoselenophenes were obtained in moderate to good yields at ambient temperature.
- This article is part of the themed collections: Organic Chemistry Frontiers HOT articles for 2018, Celebrating Excellence in Research: 100 Women of Chemistry and Celebrating Excellence in Research: Women at the Frontiers of Chemistry


 Please wait while we load your content...
Please wait while we load your content...