Asperones A–E, five dimeric polyketides with new carbon skeletons from the fungus Aspergillus sp. AWG 1–15†
Abstract
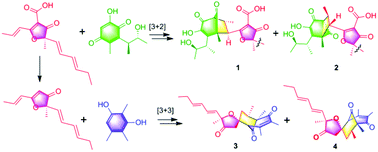
Asperones A–E (1–5), five dimeric polyketides with two distinct skeletons generated by the crucial [3 + 2] and [3 + 3] cycloadditions, were isolated from fungal metabolites of an Aspergillus sp. Their structures were established by spectroscopic data, chemical derivatization, single-crystal X-ray diffraction, and electronic circular dichroism calculations. Compounds 2–4 showed significant nitric oxide (NO) inhibition in lipopolysaccharide (LPS)-induced RAW 264.7 macrophage cells, and exhibited IC50 values of 16.0, 13.2, and 6.0 μM, respectively.
- This article is part of the themed collection: Organic Chemistry Frontiers HOT articles for 2018


 Please wait while we load your content...
Please wait while we load your content...