Heteroatom dopings and hierarchical pores of graphene for synergistic improvement of lithium–sulfur battery performance†
Abstract
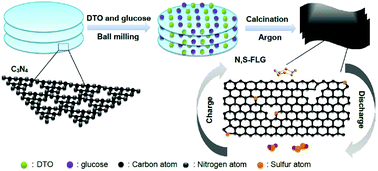
Lithium–sulfur batteries have attracted wide research interest due to their high specific capacity (1675 mA h g−1) and high specific energy (2500 W h kg−1). Herein, the two-dimensional nitrogen/sulfur synchronous-doped few-layer graphene (N,S-FLG) was prepared by a simple calcination route at 900 °C under an argon atmosphere with g-C3N4 as a structural template. The calcination temperature favorably adjusted the doping levels and surface texture of the as-obtained samples. Relying on the synergistic interaction of the structural traits, as the reservoir of sulfur, NS-FLG-900 (calcination temperature 900 °C) can maintain a reversible capacity of 602 mA h g−1 after 300 cycles at a current density of 0.8 A g−1 and 506.4 mA h g−1 after 500 cycles at a current density of 1.6 A g−1. The outstanding performance is obtained due to the synergistic effect of the introduction of N and S into the carbon lattice, the high pore volume (1.31 cm3 g−1) and large specific surface area (460.9 m2 g−1).
- This article is part of the themed collection: Inorganic Chemistry Frontiers HOT articles for 2018


 Please wait while we load your content...
Please wait while we load your content...