Selective and rapid detection of ascorbic acid by a cobalt oxyhydroxide-based two-photon fluorescent nano-platform†
Abstract
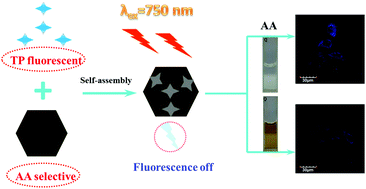
The development of an efficient and easy fabricated approach to detect ascorbic acid (AA) is of physiological and pathological significance. In this work, a two-photon sensor platform which is constituted with a 1,8-naphthalimide-based fluorophore and CoOOH nanosheets was designed in which the blue two-photon fluorescence of the fluorophore was suppressed to a remarkable extent via a FRET process between CoOOH nanosheets and the fluorophore. The fluorescence inhibition could be removed through the specific reaction of CoOOH and AA. Based on this feature, we have demonstrated the prominent sensing performance of the sensor platform, including excellent two-photon induced fluorescence properties, a convenient fabrication pathway, a specific response to AA, a wider linear range and a high stability. This fluorescence assay is capable of detecting AA in living cells and has potential for further application such as AA associated disease diagnosis.
- This article is part of the themed collection: Inorganic Chemistry Frontiers HOT articles for 2018


 Please wait while we load your content...
Please wait while we load your content...