Crystal structures, phase transitions and thermal expansion properties of NaZr2(PO4)3–SrZr4(PO4)6 solid solutions†
Abstract
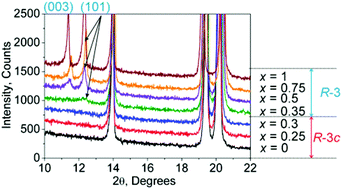
Crystal structure evolution and temperature-dependent phase transition of solid solutions are much desired for the understanding of the optimization of functional properties. Herein, the NASICON-type NaZr2(PO4)3–SrZr4(PO4)6 solid solutions with the formula Na(2−2x)Srx[ ]xZr4(PO4)6 (0 ≤ x ≤ 1), where [ ] represents the vacancy, were prepared by the sol–gel method, and their crystal structures, phase transitions and thermal expansion properties were investigated in detail. In the range of x = 0.3–0.35, there is a reversible structural phase transition R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c ↔ R
c ↔ R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) and the different structural models of the R
and the different structural models of the R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) phase and R
phase and R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c phase were built to better understand the phase transition mechanism. We determined the phase transition boundary between the R
c phase were built to better understand the phase transition mechanism. We determined the phase transition boundary between the R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c and R
c and R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) structures and predicted the phase transition temperature of Na(2−2x)Srx[ ]xZr4(PO4)6 with any x for further investigation of controlled physical properties. The results indicated that Na0.5Sr0.75[ ]0.75Zr4(PO4)6 showed a near zero thermal expansion in the temperature range of 450–600 K, which can find potential applications.
structures and predicted the phase transition temperature of Na(2−2x)Srx[ ]xZr4(PO4)6 with any x for further investigation of controlled physical properties. The results indicated that Na0.5Sr0.75[ ]0.75Zr4(PO4)6 showed a near zero thermal expansion in the temperature range of 450–600 K, which can find potential applications.
- This article is part of the themed collection: Inorganic Chemistry Frontiers HOT articles for 2018


 Please wait while we load your content...
Please wait while we load your content...