Highly selective electrochemical detection of antipsychotic drug chlorpromazine in drug and human urine samples based on peas-like strontium molybdate as an electrocatalyst†
Abstract
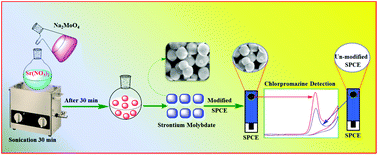
The countless use of antibiotics in veterinary and human medicine causes severe health risks to both humans and animals. In this context, monitoring of the antibiotic drug in the veterinary and human pathological system is important and provokes a universal challenge. Therefore, development of simple and sensitive inorganic materials with unique morphology is of great importance for the trace level monitoring of pharmaceutical content in the environment. Herein, we developed a novel peas-like strontium molybdate catalyst (SrMoO4; SrM) synthesized by a simple sonochemical approach and utilized as an electrochemical sensor for the detection of antipsychotic drug chlorpromazine (CPZ). The crystalline structure, surface morphology, elemental compositions and textural properties were systematically investigated by various analytical and spectroscopic techniques. As an electrochemical sensor, inorganic binary SrM modified screen printed carbon electrode (SrM/SPCE) exhibited an enhanced electrocatalytic activity towards CPZ sensing with excellent analytical performance such as wide linear response ranges and lowest detection limit of 0.1–143 and 153–1683 μM and 0.028 μM respectively. Moreover, the as-prepared SrM/SPCE showed an excellent selectivity even in the existence of co-interfering drugs, biological compounds and common metal ions. In addition, the SrM/SPCE applied to the real samples analysis in commercially available CPZ drug and human urine samples and the observed recoveries are quite satisfactory.
- This article is part of the themed collection: Inorganic Chemistry Frontiers HOT articles for 2018


 Please wait while we load your content...
Please wait while we load your content...