Functional hydrophobic and hetero-grafted block comb polymers via a combination of spontaneous zwitterionic copolymerisation and redox-initiated RAFT polymerisation†
Abstract
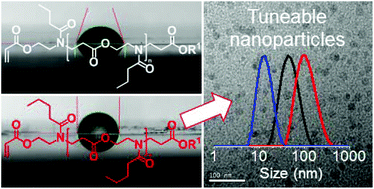
The SZWIP of 2-oxazolines enables the one-pot synthesis of N-acylated poly(amino ester) based macromonomers with tuneable hydrophobicity. Their redox-initiated RAFT (co-)polymerisation yields hydrophobic homo- or amphiphilic block comb polymers with pendant carboxy groups, which can be self-assembled into nano-sized pH-responsive aggregates. The availability for further functionalisations in combination with their non-toxicity highlights their future potential for biomedical applications.
- This article is part of the themed collection: Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...