Polymerizations in oil-in-oil emulsions using 2D nanoparticle surfactants†
Abstract
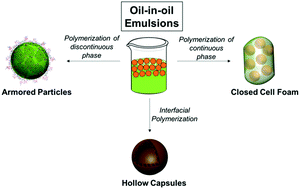
Oil-in-oil emulsions are especially attractive for compartmentalized reactions with water-sensitive monomers which cannot be used with traditional oil/water emulsions. Herein, we demonstrate that alkylated graphene oxide nanosheets can be used as a surfactant for three different oil-in-oil emulsion polymerizations and to prepare closed-cell foams, hollow capsules, and functionalizable particles.
- This article is part of the themed collection: Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...