Environmental effects of ozone depletion, UV radiation and interactions with climate change: UNEP Environmental Effects Assessment Panel, update 2017
A. F.
Bais
 a,
R. M.
Lucas
a,
R. M.
Lucas
 b,
J. F.
Bornman
b,
J. F.
Bornman
 *c,
C. E.
Williamson
*c,
C. E.
Williamson
 d,
B.
Sulzberger
e,
A. T.
Austin
f,
S. R.
Wilson
d,
B.
Sulzberger
e,
A. T.
Austin
f,
S. R.
Wilson
 g,
A. L.
Andrady
h,
G.
Bernhard
g,
A. L.
Andrady
h,
G.
Bernhard
 i,
R. L.
McKenzie
i,
R. L.
McKenzie
 j,
P. J.
Aucamp
k,
S.
Madronich
j,
P. J.
Aucamp
k,
S.
Madronich
 l,
R. E.
Neale
l,
R. E.
Neale
 m,
S.
Yazar
m,
S.
Yazar
 n,
A. R.
Young
n,
A. R.
Young
 o,
F. R.
de Gruijl
o,
F. R.
de Gruijl
 p,
M.
Norval
q,
Y.
Takizawa
r,
P. W.
Barnes
p,
M.
Norval
q,
Y.
Takizawa
r,
P. W.
Barnes
 s,
T. M.
Robson
s,
T. M.
Robson
 t,
S. A.
Robinson
t,
S. A.
Robinson
 u,
C. L.
Ballaré
u,
C. L.
Ballaré
 f,
S. D.
Flint
v,
P. J.
Neale
f,
S. D.
Flint
v,
P. J.
Neale
 w,
S.
Hylander
w,
S.
Hylander
 x,
K. C.
Rose
x,
K. C.
Rose
 y,
S.-Å.
Wängberg
y,
S.-Å.
Wängberg
 z,
D.-P.
Häder
z,
D.-P.
Häder
 aa,
R. C.
Worrest
aa,
R. C.
Worrest
 ab,
R. G.
Zepp
ac,
N. D.
Paul
ad,
R. M.
Cory
ab,
R. G.
Zepp
ac,
N. D.
Paul
ad,
R. M.
Cory
 ae,
K. R.
Solomon
ae,
K. R.
Solomon
 af,
J.
Longstreth
af,
J.
Longstreth
 ag,
K. K.
Pandey
ah,
H. H.
Redhwi
ai,
A.
Torikai
aj and
A. M.
Heikkilä
ak
ag,
K. K.
Pandey
ah,
H. H.
Redhwi
ai,
A.
Torikai
aj and
A. M.
Heikkilä
ak
aAristotle Univ. of Thessaloniki, Laboratory of Atmospheric Physics, Thessaloniki, Greece
bNational Centre for Epidemiology and Population Health, Australian National Univ., Canberra, Australia
cCurtin Univ., Curtin Business School, Perth, Australia. E-mail: Janet.Bornman@curtin.edu.au
dMiami Univ. Department of Biology, Oxford, Ohio, USA
eSwiss Federal Institute of Aquatic Science and Technology, Dübendorf, Switzerland
fUniv. of Buenos Aires, Faculty of Agronomy and IFEVA-CONICET, Buenos Aires, Argentina
gSchool of Chemistry, Centre for Atmospheric Chemistry, Univ. of Wollongong, Wollongong, Australia
hDepartment of Chemical and Biomolecular Engineering, North Carolina State Univ., Raleigh, NC, USA
iBiospherical Instruments Inc., San Diego, CA, USA
jNIWA, Lauder, Central Otago, New Zealand
kPtersa Environmental Consultants, Faerie Glen, South Africa
lNational Center for Atmospheric Research, Boulder, Colorado, USA
mQueensland Institute of Medical Research, Royal Brisbane Hospital, Brisbane, Australia
nUniv. of Western Australia, Centre for Ophthalmology and Visual Science, Lions Eye Institute, Perth, Australia
oKing's College London, London, UK
pDepartment of Dermatology, Leiden Univ. Medical Centre, Leiden, The Netherlands
qUniv. of Edinburgh Medical School, UK
rAkita Univ. School of Medicine, National Institute for Minamata Disease, Nakadai, Itabashiku, Tokyo, Japan
sDepartment of Biological Sciences and Environment Program, Loyola Univ., New Orleans, USA
tResearch Programme in Organismal and Evolutionary Biology, Viikki Plant Science Centre, Univ. of Helsinki, Finland
uCentre for Sustainable Ecosystem Solutions, School of Biological Sciences, Univ. of Wollongong, Wollongong, NSW 2522, Australia
vDept of Forest, Rangeland and Fire Sciences, Univ. of Idaho, Moscow, ID, USA
wSmithsonian Environmental Research Center, Edgewater, Maryland, USA
xCentre for Ecology and Evolution in Microbial model Systems, Linnaeus Univ., Kalmar, Sweden
yDept of Biological Sciences, Rensselaer Polytechnic Institute, Troy, NY, USA
zDept Marine Sciences, Univ. of Gothenburg, Göteborg, Sweden
aaFriedrich-Alexander Univ. Erlangen-Nürnberg, Dept of Biology, Möhrendorf, Germany
abCIESIN, Columbia Univ., New Hartford, Connecticut, USA
acUnited States Environmental Protection Agency, Athens, Georgia, USA
adLancaster Environment Centre, Lancaster Univ., LA1 4YQ, UK
aeEarth and Environmental Sciences, Univ. of Michigan, Ann Arbor, MI, USA
afCentre for Toxicology, School of Environmental Sciences, Univ. of Guelph, Guelph, ON, Canada
agThe Institute for Global Risk Research, Bethesda, MD, USA
ahInstitute of Wood Science and Technology, Bengaluru, India
aiChemical Engineering Dept, King Fahd Univ. of Petroleum and Minerals, Dhahran, Saudi Arabia
ajMaterials Life Society of Japan, Kayabacho Chuo-ku, Tokyo, Japan
akFinnish Meteorological Institute R&D/Climate Research, Helsinki, Finland
First published on 6th February 2018
Abstract
The Environmental Effects Assessment Panel (EEAP) is one of three Panels of experts that inform the Parties to the Montreal Protocol. The EEAP focuses on the effects of UV radiation on human health, terrestrial and aquatic ecosystems, air quality, and materials, as well as on the interactive effects of UV radiation and global climate change. When considering the effects of climate change, it has become clear that processes resulting in changes in stratospheric ozone are more complex than previously held. Because of the Montreal Protocol, there are now indications of the beginnings of a recovery of stratospheric ozone, although the time required to reach levels like those before the 1960s is still uncertain, particularly as the effects of stratospheric ozone on climate change and vice versa, are not yet fully understood. Some regions will likely receive enhanced levels of UV radiation, while other areas will likely experience a reduction in UV radiation as ozone- and climate-driven changes affect the amounts of UV radiation reaching the Earth's surface. Like the other Panels, the EEAP produces detailed Quadrennial Reports every four years; the most recent was published as a series of seven papers in 2015 (Photochem. Photobiol. Sci., 2015, 14, 1–184). In the years in between, the EEAP produces less detailed and shorter Update Reports of recent and relevant scientific findings. The most recent of these was for 2016 (Photochem. Photobiol. Sci., 2017, 16, 107–145). The present 2017 Update Report assesses some of the highlights and new insights about the interactive nature of the direct and indirect effects of UV radiation, atmospheric processes, and climate change. A full 2018 Quadrennial Assessment, will be made available in 2018/2019.
1 Ozone–climate interactions and effects on solar ultraviolet radiation at the Earth's surface
Measured concentrations of ozone in the upper stratosphere (altitude 35–45 km) show a statistically significant increase at mid-latitudes and the tropics since around 2000.1 This increase is consistent with the predicted recovery of stratospheric ozone resulting from decreasing concentrations of ozone-depleting substances (ODSs) in the atmosphere. Apparent increases in the total ozone column (i.e., ozone concentrations integrated over all altitudes) have been observed at most latitudes since 1996. However, these increases are not yet statistically significant because of natural variability and compounding factors, such as the buildup of greenhouse gases. The only exception is Antarctica, where a significant positive trend in total ozone has been observed.2 Decreases in UV-B radiation at the Earth's surface in response to the recovery of stratospheric ozone have not been detected yet because such changes are still masked by varying attenuation of UV radiation by ozone, clouds, aerosols, and other factors. This section provides an update on observed changes in UV radiation and several climate indicators reported during the last year. Effects on UV radiation from factors other than ozone are also discussed.1.1 Actions resulting from the Montreal Protocol continue to protect the ozone layer by controlling emissions of ozone-depleting substances and are expected to mitigate global temperature rise in the future through the phase-out of hydrofluorocarbons
Emissions of ozone-depleting substances (ODSs) in the USA have been decreasing considerably from 2008 to 2014, but hydrofluorocarbons (HFCs), which are ODS replacements with high global warming potential (e.g., HFC134a), have been increasing.3 Phase-out of HFCs by 2030 in accordance with the Kigali amendment of 2016 is projected to reduce the climate impacts of HFCs in the upper troposphere and stratosphere by 90% by the year 2050,4 and to avoid additional warming near the Earth's surface of up to 0.5 °C.51.2 Evidence that ozone over Antarctica has started to recover in both austral spring and summer has been robustly identified in the ozone profile and total column measurements of ozone, demonstrating the effectiveness of the Montreal Protocol
A new study using ozone profile data from nine stations2 has confirmed the first signs of recovery of Antarctic ozone that were reported earlier6 using data from two stations only. Statistically significant (95% confidence level) positive trends in ozone concentrations for 2001–2013 were found for austral spring (September, October, and November) in the lower stratosphere (about 10–20 km) (Fig. 1A). Trends in total column ozone inside the polar vortex were also analyzed in the new study. These trends were significant at the 95% confidence level for spring and at the 90% confidence level for summer (December, January, and February). Of note, both studies omitted data from 2015, which were probably influenced by the Calbuco volcanic aerosols, leading to a record size ozone hole in that year.7 A third study corroborated these conclusions by showing that downward trends of spring-time stratospheric ozone at the two Antarctic stations, South Pole and Syowa, for 1960–2000 have turned to upward trends for 2000–2014.8 Despite the turnaround of ozone, variability of UV-B radiation in Antarctica remains very large, with record high UV index (UVI) observed at the South Pole in spring 2015 and below average UVI in spring 2016 (Fig. 1B).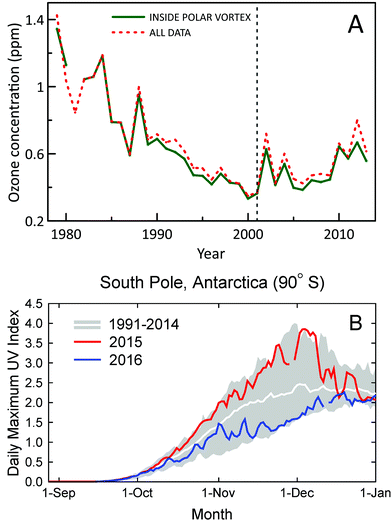 | ||
| Fig. 1 (A) Time evolution of October, November, and December average concentrations of ozone in the lower stratosphere (about 10–20 km) over Antarctica derived from ozonesonde measurements at 11 stations (red-dashed line). Average concentrations derived from measurements inside the polar vortex are shown separately (green-solid line). Figure redrawn from ref. 2. (B) Daily maximum UV Index measured at the South Pole in 2015 (red line) and 2016 (blue line) compared with the average (white line) and the lowest and highest values (grey shading) of observations performed between 1990 and 2014. Measurements between mid-October and mid-December of 2015 and 2016 were, respectively, close to the upper and lower limits of historical observations. The figure is adapted from ref. 9 and updated with data from 2016. | ||
1.3 Observations and models show that Antarctic ozone depletion influences surface climate in the tropics and the Southern Hemisphere, with effects on wind patterns, precipitation, temperature, and total solar radiation
Analysis of observations and models has shown that ozone depletion led to changes in springtime precipitation in the South Pacific Ocean, Australia, and New Zealand over the 1961–1996 period.10 These changes range from −25% to +40%, depending on location. Variability among models is large but all models indicate a consistent pattern of changes over this region. Qualitative agreement between models and measurements suggests that these effects on precipitation will likely reverse when ozone recovers in the future.Simulations by a climate model demonstrated that stratospheric ozone depletion has led to an increase in extreme precipitation and a decrease in extreme temperature over southeastern South America in the second half of the twentieth century.11 It has been suggested12 that the recently observed climate changes in the Southern Hemisphere in the summer and autumn are associated with ozone depletion, which affects circulation in the lower stratosphere and tropopause regions in the Southern Hemisphere through these seasons.
In contrast to these indirect effects, where the ozone ‘hole’ modifies circulation patterns, a modeling study13 has reported a direct effect of the Antarctic ozone ‘hole’, whereby increased UV radiation due to reduced absorption of ozone in the stratosphere contributes to total solar radiation at the surface that has increased by up to 3.8 W m−2 (∼2%) in October–December. However, most of this excess radiation is redirected upwards by the highly reflecting surface of Antarctica and does not contribute significantly to increases in temperature over Antarctica.
1.4 The recently observed depletion of stratospheric ozone in the Arctic led to increased UV radiation at the Earth's surface and contributed to changes in the surface climate of the Northern Hemisphere
Unprecedented decreases in stratospheric ozone were observed over the Arctic in winter 2010/11 due to unique meteorological conditions in the region. Less severe decreases in ozone occurred again in the winter of 2015/16,14 albeit with different timing. This recent event resulted in an increased UVI at the surface of up to 60% above the long-term average over the areas affected. However, absolute increases remained below 1 UVI unit because the event occurred early in the year when UV radiation is low.15Analysis of ozone observations in 1979–2012 revealed a statistically significant association between low values of Arctic stratospheric ozone in March and changes in climate between 30 and 70°N in March and April.16 The changes in climate include a poleward shift of the North Atlantic jet stream, lower than normal surface temperatures over eastern North America, Southeastern Europe, and Southern Asia, and higher than normal temperatures over Northern and Central Asia. Another study17 suggests that effects from variations in Arctic stratospheric ozone may extend even to the tropics and are associated with El Niño Southern Oscillation (ENSO) events.
1.5 Water vapour injected into the lower stratosphere during severe storms over the USA Great Plains might lead to chemical destruction of ozone and increased UV radiation at the surface
A modelling study18 shows that water vapour injected into the lower stratosphere during severe storms over the USA Great Plains may decrease ozone concentrations by up to 17% at altitudes between 14 and 18 km. The additional water vapour leads to hygroscopic growth of sulfate aerosols that are ubiquitously present in the stratosphere. Because the chemical reactions that lead to ozone loss are catalytically enhanced by these aerosol particles, the additional aerosol surface area provided by water vapour greatly increases the speed of these reactions, resulting in loss of ozone. The magnitude of the effect depends strongly on stratospheric temperatures at the time of the injection and is spatially limited to the area where the storm occurs. However, these effects of water vapour with respect to the total ozone column and surface UV radiation are small, and there is currently insufficient evidence to assess whether these effects will change over time. This effect on the total ozone column is less than 2% over the area of the storm. Given the small spatial and temporal extent of these events, any changes in UV radiation received at the Earth's surface would be of minimal biological importance.1.6 As changes in stratospheric ozone outside the Polar regions are small, changes in the attenuation of UV-B radiation under cloud-free skies in populated areas are mainly controlled by the concentrations of aerosols and the wavelength dependence of their optical properties
Carbonaceous aerosols resulting from combustion include black carbon (BC), which is primarily released at elevated temperatures from burning of fossil fuels, biofuels, and biomass, and brown carbon (BrC), which is produced by the burning of organic matter at lower temperatures such as by forest fires. The wavelength dependence of the absorption of UV and visible radiation by BC is relatively small. However, measurements from the ground and space at Santa Cruz, Bolivia, confirmed that the absorption by BrC has a strong wavelength dependence in the UV with the largest absorption observed at UV-B wavelengths.19 These Aerosol effects are much larger in the UV than in the visible region. By considering the different fractions of BC and BrC, the study concluded that absorption by BrC at this site caused an additional 20–25% reduction at the shortest UV-B wavelengths reaching the surface (i.e., 305 nm) compared to the BC-only absorption. If confirmed, unaccounted reduction in surface UV-B irradiance by BrC could be important for health risk assessments.Aerosols are a more crucial factor in controlling UV-B radiation than thought in the past, but available tools for quantifying their effects are still inadequate. The need for development of methods and instrumentation to quantify the absorption efficiency of aerosols at UV wavelengths already has been discussed in a previous assessment.20 Data from a multi-filter shadowband radiometer and a sun-photometer have been combined to quantify the absorption efficiency of aerosols over Athens, Greece, at selected wavelengths in the UV-A and visible range (332–1020 nm). The largest absorbing efficiency has been found for organic and dust aerosols.21 A new sun-photometer (UVPFR) developed at PMOD/WRC Davos, Switzerland, has been extensively evaluated during two campaigns in Izaña-Tenerife, Spain, in 2015 and 2016, and compared with a Brewer spectrophotometer. It was found that both instruments can measure the Aerosol optical depth (AOD) with 0.01 precision at UV-B wavelengths between 305 and 320 nm.22 Furthermore, a new method has been proposed to enable more accurate calibration of AOD sun-photometers at locations with high and variable Aerosol load.23 Such improvements will help towards clearer separation of the effects on UV-B radiation from ozone and aerosols.
1.7 When the sun is unobscured during partly cloudy conditions, UV irradiances can be higher than under clear-sky conditions
Such UV irradiance enhancements by clouds rarely exceed 20% of clear-sky values, however, and they are smaller for UV-B than for visible or UV-A irradiance. There has been confusion in the recent literature about the magnitude and wavelength-dependence of the effects of clouds on UV radiation. High-quality spectral measurements obtained from instruments at several sites covering a wide range of altitudes (up to 3.4 km at Mauna Loa Observatory in Hawaii) have demonstrated that the attenuation of UV-B radiation by clouds is, typically, slightly smaller than for UV-A radiation (cloud modification factor (CMF) < 1 in Fig. 2). However, during partly cloudy conditions when the sun is not obscured, radiation at all wavelengths can be significantly higher than for clear-sky conditions because of light scattering from clouds that are brighter than the blue sky. At such times (CMF > 1 in Fig. 2), cloud enhancements tend to be smaller in the UV-B than in the UV-A or visible regions compared with clear skies. Enhancements of UV-B radiation greater than 20% are rare, and in snow-free conditions, enhancements of UV-A radiation by clouds are less than 40%.24,25 Enhancements by clouds can be larger in the visible region, but they rarely exceed 50%.24,25 These results are consistent with earlier studies.26,27 “Cloud enhancement” events can substantially increase exposure to UV radiation for short periods, so can be important for human exposure. However, over longer periods (e.g., over the course of a day), the presence of clouds usually reduces the total dose of UV radiation.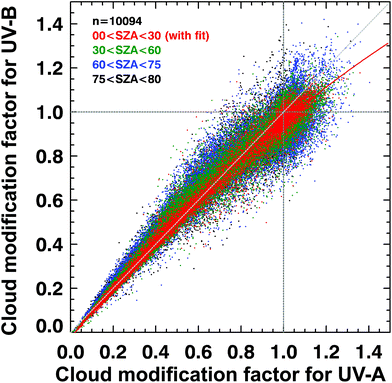 | ||
| Fig. 2 Spectral dependence of effects of clouds on solar UV radiation in terms of the cloud modification factor (CMF, defined as the ratio of measured irradiance to calculated clear-sky irradiance). The figure shows the relationship between CMF in the UV-B and UV-B regions derived from many measurements at Mauna Loa Observatory, Hawaii. Events of attenuation by clouds correspond to CMF < 1 (lower left), while events of enhancement by clouds correspond to CMF > 1 (upper right). Updated from ref. 24. | ||
1.8 Changes in UV radiation since the onset of stratospheric ozone depletion in the mid-1960s over northern mid-latitudes have been caused mainly by changes in aerosols and clouds, while decreasing stratospheric ozone had a role only up to the mid-1990s
Long-term datasets of surface UV radiation from ground-based instruments are sparse and only a few adequately cover the period since the onset of the ozone depletion. Therefore, models and ancillary data have been used to reconstruct the UV irradiance data for the past. These are often limited by the availability of ozone data derived by satellites, which started operating only in the late 1970s.One of the longest UVI series (1964–2014) was reconstructed for Belsk, Poland, from a statistical model using Aerosol extinction and total ozone data.28 Increasing aerosols caused a decline in clear-sky UVI of up to 6% between 1964 and the mid-1970s, while increases in the UVI of about 5–6% per decade in 1974–1996 were caused in equal parts by decreasing aerosols and ozone depletion. Since 1996, UVI is no longer changing, as Aerosol and ozone have been stable.
Measurements and model estimates for the Polish Polar Station, Hornsund (77°N), revealed small and non-significant positive trends (<1% per year) of daily erythemal doses for 1983–2016.29 A statistically significant trend of decreasing doses of ca. 1% per year was found for 1996–2016 in April, May, and June. This trend could not be attributed to observed increases in total ozone and was due mainly to effects of clouds.
The dominant effect on total solar radiation, resulting from decreasing cloudiness over Europe (1983–2010), has been reconfirmed from satellite data.30 The annually averaged solar irradiance was found to have increased by ca. 2 W per m2 per decade over Central and Eastern Europe. This represents an increase of less than 1% per decade, which may not be important for impacts of UV radiation, but may be important for global warming.
Statistically significant decreases in daily surface UV radiation from 1961 to 2015 were reported over most regions of China, ranging between 0.27 and 0.63 kJ per m2 per year (0.15 and 0.37% per year).31 These trends were derived from reconstructed data based on a model and proxy data from 724 weather stations, and are caused mainly by changes in aerosols, clouds, and water vapour. Trends for UV-B radiation for these stations are not available but are expected to be of similar magnitude because changes in total ozone over this time frame were comparatively small.
1.9 Accurate and consistent measurements of UV radiation from ground- and space-based systems are required for the detection of changes due to variations in ozone, aerosols, and clouds, as well as for public information
Radiometers operating unattended in harsh environments may occasionally report faulty data. Without proper quality control, such data can lead to false conclusions, as for example the extremely high UVI of 43.3 at the tropical Andes reported by Cabrol et al.32 A recent study where the data and methods were critically reviewed and incorrect data discarded, suggests that the maximum UVI at this location was in the range of 25 ± 5.33Satellite UV data are often provided at spatial resolution of tens of kilometers. A method has been proposed to scale the UVI data provided by the space-borne Ozone Monitoring Instrument (OMI) down to 1 × 1 km grid. This downscaling was achieved by interpolation of satellite data and other measurements (e.g., surface albedo, Aerosol optical depth, cloud cover, dew point, ozone, surface incoming shortwave flux, and sulfur dioxide).34 Such higher resolution data can be more useful in exposure studies where UV radiation data at specific locations are needed.
Images from smartphone cameras have been tested as UV monitoring devices for improved personalisation and public awareness of exposure to UV radiation. Currently the accuracy of these devices is much lower than scientific-grade UV sensors in use, either due to poor technical characteristics and calibration,35 or due to inappropriate measurement principles.36 Data from such devices should be used with caution for information on actual sun-burning radiation levels, but may be helpful in public health campaigns pending further evaluation.
2 Ultraviolet radiation and human health in a changing climate
The main adverse health effects of higher exposure to UV-B radiation, which is, in part, a consequence of depletion of stratospheric ozone, are increased risks of skin cancers, immune suppression, and disorders of the eye, including cataract. Here we assess recent evidence regarding these adverse effects, consider factors related to sun protection, and evaluate new evidence of the beneficial effects of sun exposure. Expected recovery of stratospheric ozone because of the Montreal Protocol and its amendments, and lower solar radiation in some regions due to increased cloud cover, will reduce ambient UV-B radiation in the future. It is thus important to understand both the risks and benefits of exposure to solar radiation.2.1 The incidence of cutaneous malignant melanoma continues to increase in many countries, but is highly variable within and between countries due to differences in ambient UV radiation, skin type, and behaviour in relation to exposure to the sun
The incidence of cutaneous malignant melanoma (CMM) is continuing to increase in many countries but the rate of increase is variable. For example, incidence of CMM increased by ca. 4% per year in Estonia (1995 to 2013)37 but increased by only ca. 0.6% per year over a recent 7-year period in the USA (2009–2016).38 In Estonia, the rapid increase in incidence of CMM began following the country's transition to an open market economy in the 1990s, and may have been driven by increased use of tanning beds and holidays in sunny locations.37New data from the Global Burden of Disease Study39 show the overall burden to health systems of new cases of CMM (Fig. 3). Data for overall incidence, and trends in incidence, conceal variation according to age, sex and ethnicity. For example, the increase in incidence of CMM in Estonia was particularly high in younger women (<50 years): an increase of 6% per year from 1995–2013 compared to 2% per year for women aged 50–64 years, 3% per year for women ≥65 years, and 4.4% for men of all ages.37 Of note, in younger men, there was a 12% per year increase in incidence of CMM during 2005–2013, compared to an increase of 0.6% per year from 1995–2005. In the USA, the incidence of CMM was considerably lower in Hispanics (4.2 per 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) than non-Hispanic whites (22.6 per 100
000) than non-Hispanic whites (22.6 per 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) in 2012; the incidence significantly decreased in Hispanics between 2003 and 2012 (average annual decrease of 1.4%),40 but significantly increased in non-Hispanic whites over a similar time period (average annual increase 1.7% from 2002–2011).41 Diagnosis is often at a later stage and with greater tumour thickness in Hispanics compared to non-Hispanic whites, resulting in poorer survival.40 Notably, Hispanics who have adopted characteristics of a US lifestyle and US-born Hispanics have a higher risk of sunburn and CMM compared to those who retain their traditional lifestyles.40 CMM is uncommon in African Americans. When it occurs, it is most often found on the sole of the foot, and by the time medical attention is sought is often deeper and at a more advanced stage and thus more likely to be lethal.42
000) in 2012; the incidence significantly decreased in Hispanics between 2003 and 2012 (average annual decrease of 1.4%),40 but significantly increased in non-Hispanic whites over a similar time period (average annual increase 1.7% from 2002–2011).41 Diagnosis is often at a later stage and with greater tumour thickness in Hispanics compared to non-Hispanic whites, resulting in poorer survival.40 Notably, Hispanics who have adopted characteristics of a US lifestyle and US-born Hispanics have a higher risk of sunburn and CMM compared to those who retain their traditional lifestyles.40 CMM is uncommon in African Americans. When it occurs, it is most often found on the sole of the foot, and by the time medical attention is sought is often deeper and at a more advanced stage and thus more likely to be lethal.42
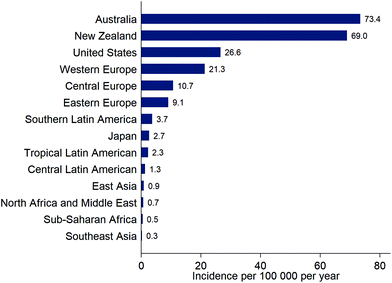 | ||
| Fig. 3 Estimates of the incidence (new diagnoses) of cutaneous malignant melanoma for selected locations, from the Global Burden of Disease Study, 201639 (note that these estimates are not adjusted for the differing age distributions of the populations). | ||
In South and Central America, the age-standardised incidence rate for CMM (across varying time periods, mainly 2003–2007) ranged from 1 to 5 per 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and tended to be higher further from the Equator.43 European ancestry was an important risk factor for CMM. Incidence rates were relatively stable from 1985–2007 except in Chilean males where there was an increase of 10% annually (the equivalent increase was 1.6% in women, suggesting that this is not an artefact of changes in reporting). The strong skin tanning culture in Brazil probably explains the high incidence of CMM (4.9 per 100
000 and tended to be higher further from the Equator.43 European ancestry was an important risk factor for CMM. Incidence rates were relatively stable from 1985–2007 except in Chilean males where there was an increase of 10% annually (the equivalent increase was 1.6% in women, suggesting that this is not an artefact of changes in reporting). The strong skin tanning culture in Brazil probably explains the high incidence of CMM (4.9 per 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) compared to other South or Central American countries (e.g., 1.9 per 100
000) compared to other South or Central American countries (e.g., 1.9 per 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 in Costa Rica).43
000 in Costa Rica).43
Although CMM is uncommon in Japan (Fig. 3), the skin was the most frequent body site (81%) for melanoma in a recent analysis of a national data-base, followed by mucosal melanoma (15%), uveal melanoma (2.9%) and melanoma of unknown origin (1.8%).44 The most common site for CMM was the lower limb (49%), followed by the upper limb (17%), trunk (12%), and head and neck (6%). This compares to the relative proportions for the USA (1988–2010) of CMM (96%), uveal melanoma (2.6%), and mucosal melanoma (1.2%).45 Distribution on skin sites in the USA (1999–2006) was lower limb (33%), upper limb (27%), trunk (26%), and head and neck (14%).46 In Tunisia, the incidence of CMM is very low (ca. 0.6 per 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 per year) and most of these tumours occur on skin surfaces that are not exposed to the sun, such as the sole of the foot.47
000 per year) and most of these tumours occur on skin surfaces that are not exposed to the sun, such as the sole of the foot.47
A recent economic analysis from Australia that included costs of diagnosis and treatment of CMM, as well as management of lesions subsequently diagnosed as benign, found the estimated cost of CMM to be AUD$272 million per year.48 The high and increasing incidence of CMM, coupled with increasingly effective but expensive immunotherapies, means that programs promoting personal sun protection, including those with a specific focus on prevention of CMM, are very important.
2.2 Keratinocyte cancers (previously called non-melanoma skin cancer and comprising squamous cell carcinoma and basal cell carcinoma) cause a substantial health burden, and there is evidence of increasing incidence in many countries
Monitoring the incidence of skin cancer continues to be hampered by lack of adequate registration, exemplified by a recent study from the United Kingdom that found only about two-thirds of cutaneous squamous cell carcinomas (SCC) identified through pathology laboratories were included in the cancer registry.49 Allowing for this under-registration and extrapolating to the wider United Kingdom population, it was estimated that, excluding basal cell carcinoma (BCC), SCC was the 5th most common of the potentially lethal cancers (after bowel, prostate, lung and breast cancers). In many countries, there is evidence that the incidence is increasing. For example, in the eastern region of the United Kingdom between 2003 and 2012 there was a 2.8-fold increase in the incidence of SCC lesions.49 Based on data from this region, it was predicted that, for the whole of the United Kingdom, there would be 298![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 308 cases of BCC and 81
308 cases of BCC and 81![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 694 cases of SCC in 2025.50 The estimated costs (in 2025, excluding patient costs, lost productivity, and premature mortality) of these skin cancers ranged from £338 to £465 million. An analysis of data from Girona (Spain) found an average annual increase between 1994 and 2012 of 1.6% for SCC and 1.5% for BCC, but the pattern differed depending on sex. For SCC, the yearly increase was similar for men and women (1.6% and 1.4%, respectively) whereas, for BCC, increase in incidence was twice as high in women compared to men (2.0% vs. 1.0%).51 In Minnesota, USA, there was an increase in the incidence of around 1.5-fold for BCC and 2.6-fold for SCC between the early 1980s and the first decade of the 2000s.52 These changes most likely reflect an increase in sun-seeking behaviours rather than higher levels of ambient UV-B radiation.
694 cases of SCC in 2025.50 The estimated costs (in 2025, excluding patient costs, lost productivity, and premature mortality) of these skin cancers ranged from £338 to £465 million. An analysis of data from Girona (Spain) found an average annual increase between 1994 and 2012 of 1.6% for SCC and 1.5% for BCC, but the pattern differed depending on sex. For SCC, the yearly increase was similar for men and women (1.6% and 1.4%, respectively) whereas, for BCC, increase in incidence was twice as high in women compared to men (2.0% vs. 1.0%).51 In Minnesota, USA, there was an increase in the incidence of around 1.5-fold for BCC and 2.6-fold for SCC between the early 1980s and the first decade of the 2000s.52 These changes most likely reflect an increase in sun-seeking behaviours rather than higher levels of ambient UV-B radiation.
The most recent Global Burden of Disease Study found that cutaneous SCC contributed 0.06% to the total disease burden, as measured in disability-adjusted life years (DALYs), a measure which incorporates years of healthy life lost to death as well as disability.39 BCC contributed less than 0.01% due to extremely low mortality for this cancer. At a population level, the main impact of KCs relates to the high overall cost of managing the very large numbers of these lesions.53
2.3 The relative proportions of the types of skin cancer in African and Middle Eastern countries differ to that of Western countries
In predominantly fair-skinned populations in Western countries, the most common type of skin cancer is BCC (71% of KC in Australia; 80% of KC in United Kingdom).54 Of the other UV-induced skin cancers – SCC, CMM, and Merkel cell carcinoma – SCC is the most common, followed by CMM; Merkel cell carcinoma is rare.55 In contrast to this pattern, in a study from the Northern Cape Province of South Africa, a review of histopathological skin cancer data showed that SCC was the commonest cancer (45.4%), followed by BCC (27.8%), and CMM (3.1%) (with Kaposi's sarcoma contributing the remaining 6.5%).56 Skin cancer was the 9th most common malignancy in Saudi Arabia in 2010 (3.2% of newly diagnosed cancers).57 The commonest tumour was BCC (36%), followed by SCC (23%); CMM made up 7% of skin cancers (other skin cancers contributed the remaining 34%). Importantly, there is a lack of high quality population-based data from many countries making it difficult to monitor trends in incidence.2.4 Skin cancers account for 40–50% of the cancers that occur following solid organ transplantation, occurring almost invariably on sun-exposed body sites and have a higher risk of death than in the general population
Medical management following organ transplantation requires maintenance of immune suppression to prevent rejection of the transplant. This immune suppression greatly increases the risk of UV-induced skin cancers. Risks are particularly high in older people and men, in those who have had a pre-transplant skin cancer, in regions of the world where there is high ambient UV radiation, and on body sites that are frequently exposed to UV radiation. The most common tumour is SCC, with an incidence of 65- to 250-times higher than in the general population. The incidences of BCC (10–16-fold) and of CMM (up to 8-fold) are also increased compared to the general population.58 In a study from the USA, the mortality rate from skin cancer in organ transplant recipients (35.3 per 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 person years) was nearly 10 times higher than that seen in the general population (4 per 100
000 person years) was nearly 10 times higher than that seen in the general population (4 per 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000).59 An increased risk of CMM and KC also occurs in people who have impaired immunity for other reasons, such as during chronic haemodialysis.60,61 These susceptible populations need carefully targeted advice about prevention and screening.
000).59 An increased risk of CMM and KC also occurs in people who have impaired immunity for other reasons, such as during chronic haemodialysis.60,61 These susceptible populations need carefully targeted advice about prevention and screening.
2.5 “Normal” sun-exposed skin contains thousands of mutations, including those in genes important to cancer development, but a new study shows that non-mutated cells within the epidermis eliminate mutated cells providing dynamic repair and regeneration
Skin cancers have the highest mutation burden of any cancer.62 We have previously reported that biopsies of aged, sun-exposed (but otherwise normal) skin contained thousands of evolving clones of abnormal cells in which over a quarter of the cells contained cancer-causing mutations.63 A recent study using innovative imaging techniques has shown that normal (non-mutated) cells actively eliminate mutated cells in the epidermis, and replace the abnormal clones with normal skin architecture.64 This work further elucidates our understanding of the processes involved in the genesis of skin cancers.2.6 There is little evidence that screening the general population for skin cancer reduces deaths due to skin cancer
A review of the large population screening program in Germany shows limited evidence that screening reduces mortality.65,66 However, selective screening of patients at high risk of skin cancer by primary care physicians may increase early detection of skin cancers.67 In Belgium, a total body skin examination offered to the general adult population was more cost-effective than lesion-directed screening, but both incurred costs of over USD$20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 for a gain of one quality adjusted life year (QALY).68 Notably, full skin examination is required to avoid missing a treatable CMM; 1 in 3 CMM would be missed without full skin examination.69 Screening has potential harms such as psychological distress and unnecessary removal of non-malignant lesions. In a systematic review of the evidence for screening for skin cancer, the US Preventative Services Task Force concluded that there was currently insufficient evidence to assess the balance of benefits and harms of skin cancer screening by clinicians using a visual skin examination.70
000 for a gain of one quality adjusted life year (QALY).68 Notably, full skin examination is required to avoid missing a treatable CMM; 1 in 3 CMM would be missed without full skin examination.69 Screening has potential harms such as psychological distress and unnecessary removal of non-malignant lesions. In a systematic review of the evidence for screening for skin cancer, the US Preventative Services Task Force concluded that there was currently insufficient evidence to assess the balance of benefits and harms of skin cancer screening by clinicians using a visual skin examination.70
2.7 Despite awareness of the health risks of sun exposure, there is inadequate adoption of sun protection behaviours
Although evidence suggests that multi-component community-wide interventions can reduce exposure to UV radiation,71 inadequate adoption of sun protection strategies continues. High-dose exposure to UV radiation in childhood is a major risk factor for CMM. Nevertheless, in a nationally representative sample of schools in the USA, sun safety practices and policies were uncommon.72 For example, only 12% of high schools, 18% of middle schools and 15% of elementary schools scheduled outdoors activities to avoid times when the sun was at peak intensity. In an analysis of the national database of emergency room admissions in 2013 in the USA, there were an estimated 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 826 sunburn-associated emergency room visits at an estimated cost of USD$11.2 million.73 The most commonly affected population groups were men younger than 18 years and women aged 18–29 years. Australian adolescents professed a desire to tan to achieve the perceived social benefits of being considered attractive to their peers, despite being aware of the long-term health risks.74 A study of young adults (18–34 years) showed that people who frequently used sunbeds for indoor tanning were also more likely to report never or seldom using sun protection when outdoors.75 However, a study of global trends for Google search entries from 2004–2016 showed an increase in “sunscreen” coupled with a decline in “tanning bed”,76 perhaps indicating increased awareness of the risks of sun exposure over this time period.
826 sunburn-associated emergency room visits at an estimated cost of USD$11.2 million.73 The most commonly affected population groups were men younger than 18 years and women aged 18–29 years. Australian adolescents professed a desire to tan to achieve the perceived social benefits of being considered attractive to their peers, despite being aware of the long-term health risks.74 A study of young adults (18–34 years) showed that people who frequently used sunbeds for indoor tanning were also more likely to report never or seldom using sun protection when outdoors.75 However, a study of global trends for Google search entries from 2004–2016 showed an increase in “sunscreen” coupled with a decline in “tanning bed”,76 perhaps indicating increased awareness of the risks of sun exposure over this time period.
2.8 Several studies show that comprehensive sun protection programs are cost-effective
A recent update of an earlier economic analysis in Australia showed that an additional investment of AUD$0.16 per capita per year in skin cancer prevention (with a total program cost over 20 years of AUD$62 million) could result in the prevention of 140![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cases of skin cancer and 6200 premature deaths from 2011 to 2030.77 The projected annual expenditure for 2015 in Australia for KC alone78 (including costs of diagnosis, treatment and pathology) was AUD$700 million (with costs for CMM already noted in section 2.1). Analysis of past achievement of the SunSmart program in Australia showed that, for an investment of AUD$0.37 per capita per year, 43
000 cases of skin cancer and 6200 premature deaths from 2011 to 2030.77 The projected annual expenditure for 2015 in Australia for KC alone78 (including costs of diagnosis, treatment and pathology) was AUD$700 million (with costs for CMM already noted in section 2.1). Analysis of past achievement of the SunSmart program in Australia showed that, for an investment of AUD$0.37 per capita per year, 43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 skin cancers were prevented between 1988 and 2010 at a net cost saving of AUD$94 million (based on costs of treatment averted).79 The cost of skin cancer treatment in public hospitals was 30 times higher than the funding for skin cancer prevention. These studies are consistent with previous work from Belgium,80 which also showed the cost-benefit of prevention programs, and suggest that there should be increased investment in programs that promote sun protection.
000 skin cancers were prevented between 1988 and 2010 at a net cost saving of AUD$94 million (based on costs of treatment averted).79 The cost of skin cancer treatment in public hospitals was 30 times higher than the funding for skin cancer prevention. These studies are consistent with previous work from Belgium,80 which also showed the cost-benefit of prevention programs, and suggest that there should be increased investment in programs that promote sun protection.
2.9 The pathways and effects of modulation of the immune system following exposure to solar radiation continue to be elucidated
Exposure of the skin and eyes to UV radiation modulates immune function through a range of pathways (reviewed in ref. 81). New research suggests that there may be additional pathways. Exposure of the skin to UV radiation may change the skin microbiome – the composition of the populations of bacteria, fungi, viruses, and mites that normally reside on or within the skin (e.g., about 100 million bacteria are present per cm2 of skin surface).82 However, any downstream effects of this on UV-induced immune suppression are not yet clear. Exposure to UV-B radiation alters the expression of immune-related genes, resulting in changes to the levels of cytokines in the blood,83–85 presumably through epigenetic mechanisms,83–85 and downregulation of immune pathways.85 It is likely that there is considerable cross-talk between the different pathways that influence immune function.83 There are both risks and benefits of UV-induced immune modulation, and the balance of these is an area of active research. Clarification of these issues will help to inform the development of evidence-based public health strategies regarding exposure to the sun.2.10 There is new evidence to suggest that exposure to UV radiation increases the risk of melanomas of the eye that affect the conjunctiva, but not those affecting the deeper structures of the eye (i.e., uveal melanomas)
Although rare (e.g., 0.4 per million population per year from 1973 to 2012 in the USA), the incidence of conjunctival melanoma is increasing in parallel with the increasing incidence of CMM.86 In contrast, the incidence of uveal melanoma remains relatively constant (ca. 5 per million population per year in the USA). Uveal melanomas resemble melanocytic tumours of the central nervous system, whereas conjunctival melanomas show mutation patterns similar to those seen in CMM.87,88 A recent meta-analysis found insufficient studies to assess an association between uveal melanoma and most measures of past sun exposure (such as photokeratitis, outdoor vacationing, blistering sunburns, or lifetime exposure to UV radiation). However, the evidence indicated no association between risk of uveal melanoma and outdoor leisure activity, occupational sun exposure, or latitude of birth, but increased risk in association with markers of UV sensitivity such as atypical and common cutaneous naevi (moles), freckles in the iris, and light eye colour.89 The increased risk of conjunctival melanoma related to exposure to UV radiation emphasises the importance of incorporating eye protection messages into public health campaigns.2.11 UV-induced damage to the superficial structures of the eye may provide useful biomarkers of past sun exposure
Iris freckles are small flecks of pigment on the anterior surface of the iris. Like skin freckles, iris freckles are formed because of accelerated growth of melanocytes containing large granules of melanin. A recent study suggests that iris freckles are a marker of high sun exposure to the eye.90 Evidence includes the usual location of iris freckles in the parts of the iris that are least protected from overhead solar radiation by the eyebrow and upper eyelid, and a greater number of iris freckles in people reporting a history of severe sunburn and greater number of lifetime sunburns.Conjunctival autofluorescence (CUVAF) appears to reflect sun exposure over at least several months and possibly over a lifetime of chronic exposure.91 The area and intensity of CUVAF increases with less frequent use of sunglasses,92 and larger areas occur in Caucasian children with fairer pigmentation, lighter eye and hair colour, greater number of lifetime sunburns, freckling by the end of the previous summer, and less use of sunhats.93 Further studies are required to assess the value of iris freckles and CUVAF as tools for measuring the exposure of an individual to UV radiation across a range of different environments and timeframes. The availability of objective measures of sun exposure will facilitate research into the risks and benefits of exposure to the sun.
2.12 Any link between sun exposure and age-related macular degeneration – a leading cause of blindness worldwide – remains unclear
Age-related macular degeneration (AMD) is responsible for 5% of blindness worldwide94 and is the leading cause of blindness (accounting for 54% in white Americans) in adults aged 40 years and over in European-derived populations.95 The disease burden of AMD is increasing: the age-standardised disability-adjusted life years per 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 population increased from 5.3 to 6.3 between 1990 and 2016.39 It is therefore important to understand the causes of this condition. However, the association (if any) between sun exposure and AMD remains unclear. In a recent study, working outdoors was associated with late- but not early-stage AMD.96 In a large cross-sectional European study, there was a modest increased risk of late-stage AMD in association with vitamin D deficiency. However, reverse causality – where the impaired vision resulting from AMD caused reduced sun exposure and thus vitamin D deficiency – could not be excluded.97 Improved biomarkers of ocular sun exposure may help to elucidate the association between sun exposure and AMD in the future.
000 population increased from 5.3 to 6.3 between 1990 and 2016.39 It is therefore important to understand the causes of this condition. However, the association (if any) between sun exposure and AMD remains unclear. In a recent study, working outdoors was associated with late- but not early-stage AMD.96 In a large cross-sectional European study, there was a modest increased risk of late-stage AMD in association with vitamin D deficiency. However, reverse causality – where the impaired vision resulting from AMD caused reduced sun exposure and thus vitamin D deficiency – could not be excluded.97 Improved biomarkers of ocular sun exposure may help to elucidate the association between sun exposure and AMD in the future.
2.13 New health risks are linked to exposure to solar radiation
In addition to the UV-induced skin cancers and eye diseases, there is emerging evidence of links between exposure to the sun and an increased risk of several other disorders, including diseases of the thyroid, Parkinson's disease, and mania. Recent studies support an association between greater number of naevi, past history of melanoma, or higher ambient UV radiation at the latitude of residence, and increased risk of goitre and thyroid cancer.98 Both animal99 and human studies100 show an increased risk of Parkinson's disease in association with a past history of melanoma, and vice versa. Genetic variants leading to red hair and fair skin may increase risk of both melanoma and Parkinson's.99 Seasonal affective disorder is well described; depression occurs during winter, possibly caused by low visible light (particularly blue light) leading to low serotonin levels. The opposite effect has recently been described for mania, a state of euphoria and/or overactivity. Hospital admissions for mania in Denmark were highest during summer and when levels of UV radiation were higher.101 Further work is required to verify whether these population-level associations between disease risks and levels of ambient UV radiation translate into alterations in individual risk.2.14 Sunscreens are effective in reducing the hazards of exposing the skin to UV radiation, although the sun protection factor (SPF) of a sunscreen may overestimate the protection from natural solar radiation, giving a false sense of safety
Epidemiological evidence supports the use of sunscreens to inhibit keratinocyte cancers (KC) and CMM (reviewed in ref. 102). Similarly, laboratory studies show that sunscreens inhibit the formation of cyclobutane pyrimidine dimers (CPD) in the human epidermis in vivo (reviewed in ref. 103). CPDs can be regarded as biomarkers of the DNA damage that may lead to skin cancer, at least for KC. In addition, daily use of a broad-spectrum sunscreen (providing protection across both UVA and UVB wavelengths) significantly reduced the clinical signs of photoageing.104The SPF is an internationally standardised relative measure of the ability of sunscreen, applied at 2 mg cm−2, to prevent erythema (sunburn) occurring from a single exposure to solar simulated radiation (SSR).† For example, unprotected skin receives 100% of the dose of UV radiation to the skin. Correct application of an SPF 30 sunscreen will reduce that dose of erythemally weighted UV radiation to 3% (see Fig. 4). An international study of 261 “dermatology experts” demonstrated a lack of understanding of the relationships between SPF and the percentage of sunburning UV radiation that reaches the skin.105 This lack of knowledge could result in experts giving members of the public misleading advice about photoprotection.
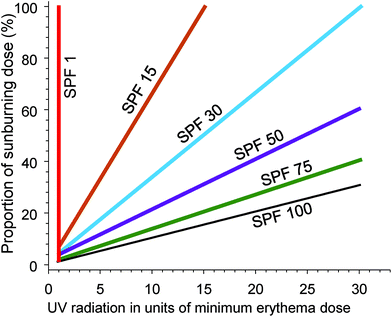 | ||
| Fig. 4 The graph shows the effectiveness of sunscreen of different sun protection factors (SPF) for preventing sunburn. The dose of UV radiation (x-axis) is presented in units of the dose that will cause minimal erythema (MED) of the skin. The y-axis is the percentage of a sunburning dose (1 MED) that will be received by the skin, using sunscreens of different SPF. With no sunscreen (SPF = 1) a dose of UV radiation of 1 MED results in 100% of the dose required to cause sunburn. With successively higher SPF sunscreens, the dose of UV radiation required to reach 1 MED (100% of a sunburning dose) increases. Adapted from ref. 106. | ||
The SPF is primarily a measure of protection against UV-B radiation; for any specific SPF, increasing protection from UV-A radiation (i.e., broad spectrum protection) reduces the degree of protection against UV-B radiation.107 Since UV-B radiation is considered to be more carcinogenic than UV-A, this may reduce protection against skin cancer, although protection from erythema will be maintained. The SPF as measured using SSR overestimates protection under natural solar radiation, possibly due to a contribution of visible radiation (not present in SSR) to erythema.108 These factors, coupled with poor coverage (see section 2.15), mean that the stated SPF may not provide the protection from solar radiation that people expect when they apply sunscreen.
2.15 The use of higher SPF sunscreens has increased in the past several decades, but correct application remains problematic and high SPF sunscreens do not provide full protection during extended exposure
In studies of beachgoers in Denmark in 2016, the frequency of sunscreen use increased from 45% in 1997 to 78% in women, and from 39% to 49% in men.109 For both men and women the median SPF of the sunscreen used increased from SPF 5 in 1997 to SPF 20 in 2016. The estimated quantity of sunscreen applied increased from 0.48 mg cm−2 in 1992 to 0.57 mg cm−2 in 2016, which is still considerably lower than that used for SPF determination. Poor application of sunscreen is a common problem, as was demonstrated in a study of 52 healthy adults (see Fig. 5);110 on average 11% of the body surface was not covered at all.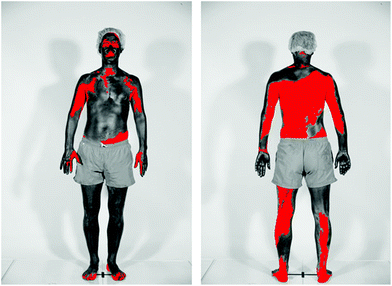 | ||
| Fig. 5 The figure shows the skin coverage following application of sunscreen. Body areas covered in sunscreen appear dark, while the red colour shows skin surfaces not covered by sunscreen. The photographs were taken using standardised UV photography (UVP) that is sensitive only to the UV-A part of the spectrum. The sunscreen used absorbs incoming UV-A radiation. Therefore, body areas covered with these UV-A filters appear dark in UVP images. (Photograph from ref. 110 reproduced with permission). | ||
A clinical trial in Texas compared sunburn in 81 people randomised to use either a beach umbrella (that had no measurable UV transmission) or a sunscreen with an SPF of 100, while outdoors for 3.5 hours at peak solar elevation in late summer.111 It was estimated that the sunscreen was applied at the recommended thickness of 2 mg cm−2, through repeated re-application. There was significantly more sunburn in the umbrella group (75% compared to 25% of the sunscreen group, P < 0.001), but participants in both groups were sunburned, demonstrating that, even with a very high SPF sunscreen, more than one strategy is necessary for optimal photoprotection.
2.16 There is growing understanding of health benefits of sun exposure that are independent of production of vitamin D
While the benefits of sun exposure to avoid vitamin D deficiency are clear, exposure to the sun appears to have a range of other beneficial effects. UV-B and UV-A radiation are absorbed by many molecules in the skin, causing chemical reactions with a wide range of sequelae. High blood pressure is the risk factor responsible for the greatest loss of disability-adjusted life years (DALYs) globally. Both observational and intervention studies support a benefit of sun exposure for high blood pressure, possibly through UV-A-mediated release of nitric oxide stores in the skin that cause arterial vasodilation and reduction in blood pressure.112,113 Exposure to UV-B radiation might inhibit the development and progression of atherosclerosis by decreasing inflammation (see section 2.9).114 Several studies suggest that low exposure to the sun or total avoidance of exposure to the sun are associated with higher rates of all-cause mortality, mainly related to increased risk of death from cardiovascular and other non-cancer diseases (reviewed in ref. 115). In mice, exposure of skin to UV-B radiation increased the concentrations in the brain and plasma of a range of neuroendocrine hormones that have wide-ranging effects on appetite, metabolism, and immune function.116 While there are many plausible pathways whereby exposure to solar radiation may have benefits for health, further research is required to establish whether such benefits truly occur, and to quantify the size of any effects.2.17 Observational studies and randomised controlled trials testing the association between vitamin D and disease-related risks have contradictory findings
Exposure to the sun is the major source of vitamin D for much of the world's population. An individual's vitamin D status is usually assessed by measuring the concentration in blood (serum or plasma) of a metabolite of vitamin D, 25-hydroxyvitamin D (25(OH)D). Meta-analyses of observational cohort studies continue to show associations between low concentrations of circulating 25(OH)D and increased risk of a wide range of health outcomes such as colorectal cancer,117 cardiovascular disease,118 dementia,119,120 adverse pregnancy outcomes,121 and childhood asthma.122 Meta-analyses of supplementation trials, however, have mostly failed to show any benefit of supplementation,123 with the possible exception of acute respiratory illnesses.124 There are several possible reasons for this discrepancy. Firstly, the results from the observational studies may have arisen due to reverse causality (the disease caused the low level of 25(OH)D rather than the low level of 25(OH)D causing the disease) or differences in other lifestyle factors that alter the disease risk and are also associated with the level of 25(OH)D (confounding). Secondly, it may be that higher concentration of 25(OH)D in blood is a marker of higher exposure to UV radiation, and it is the non-vitamin D effects of sun exposure that confer the benefit. Finally, the trials may be flawed (e.g., recruitment of people who do not have vitamin D deficiency, supplementation at the wrong life stage, with too small a dose, or for too short a time).One possible way of disentangling the divergent results is to assess associations between genetic variants associated with 25(OH)D concentration and disease risk, using an approach called Mendelian randomisation analysis.‡ Studies using this approach suggest possible causal associations between low 25(OH)D and ovarian cancer,125 multiple sclerosis,126,127 and Alzheimer's disease.128 No association was found for asthma or atopic dermatitis,129,130 cardiovascular disease,131,132 Parkinson's disease133 or schizophrenia.134 However, due to the assumptions underpinning Mendelian randomisation studies, positive findings make an important contribution but do not provide ‘proof’ of a causal relationship and the null findings may be due to inadequate sample size.
2.18 Considerable variation occurs between individuals in production of vitamin D following exposure to UV radiation and the half-life of 25(OH)D depends on its starting concentration
In one laboratory study of 22 people with similar skin types who were exposed to identical UV irradiation protocols, the maximum concentration of 25(OH)D in serum ranged from 85–216 nmol L−1.135 Similar variability has been previously described in a group of 120 adults of similar skin type in Manchester, United Kingdom, who received a 6-week course of UV irradiation. The increase in concentration of 25(OH)D in serum ranged from 5–80 nmol L−1.136The half-life of serum 25(OH)D during winter in Denmark has been shown to be dependent on the starting level.137 That is, in the group with the highest starting level (mean 25(OH)D = 132 nmol L−1, n = 22) the half-life was 89 days; in a group where the mean baseline level was 65 nmol L−1 (n = 14) the half-life was 120 days; and in a group with a low baseline 25(OH)D level (mean 25(OH)D = 43 nmol L−1, n = 92), the half-life was 199 days. These half-lives are longer than was previously thought and could be adequate to provide sufficient 25(OH)D for the duration of winter months in some locations. These findings have implications for advice regarding the target concentration of 25(OH)D at the end of summer to avoid vitamin D deficiency during winter.
2.19 In some countries there is insufficient UV-B radiation for vitamin D production during certain parts of the year and vitamin D supplementation may be required to avoid deficiency
Severe vitamin D deficiency can cause rickets in children and osteomalacia (soft bones) in adults. Modelling of UV irradiance over Europe shows that there is insufficient UV-B radiation to produce vitamin D for up to 8 months of the year in parts of Norway, and for at least 4 months in Germany, Ireland, the United Kingdom, Denmark, Finland, and Iceland.138 Thus, attention has turned to avoiding vitamin D deficiency via fortification of food. Widespread food fortification is an alternative to supplementation and, in Finland, fortifying liquid milk products resulted in a decrease in the prevalence of deficiency (25(OH)D < 30 nmol L−1) among people not using supplements from 13% to less than 1%, showing the benefit of this strategy.139 The United Kingdom Scientific Advisory Committee on Nutrition (SACN) recently concluded that United Kingdom residents should be advised to routinely take 400 IU of supplementary vitamin D per day to avoid severe vitamin D deficiency.2.20 Evidence is accumulating that shows lack of exposure to the sun in teen years increases the risk of myopia (nearsightedness)
In an elderly European population (mean age 72 years), higher exposures to UV-B radiation (measured using self-reported sun exposure information and meteorological data) in adolescence and young adulthood were associated with a reduced risk of being myopic.140 Furthermore, myopic children who wore contact lenses that transmitted radiation at 360–400 nm (UV-A wavelengths) in addition to visible radiation had less progression of myopia over one year than children wearing contact lenses or glasses that blocked the UV-A wavelengths.141 Experimental studies in chicks suggest that exposure to these longer UV wavelengths suppresses elongation of axial length of the eye, and thus development of myopia.141 There are, however, limitations to these studies, including differences in the transmission of UV radiation through the eyes of chicks compared to those of humans.142 Understanding the wavelength dependence of the induction of myopia is important in view of the known risks of exposure of the eye to UV wavelengths.2.21 Darker skin pigmentation moderates both the beneficial and deleterious effects of UV radiation, but the magnitude of these effects is unclear
Darker skin pigmentation indicates a greater amount of melanin in the epidermis. Melanin is a natural sunscreen but quantification of its protection against the acute and long-term adverse effects of solar radiation remains controversial.143 There is also controversy about the inhibitory effect of melanin on production of vitamin D. A field study of children in India showed a greater increase in concentration of 25(OH)D in serum following exposure to the sun in children with lighter skin type (skin type IV) compared to those with darker skin type (skin type V).144 In contrast, in a small study of post-menopausal women (Caucasian, n = 9, skin types II/III; South Asian, n = 8, skin types IV/V) given an identical UV irradiation protocol, there were no skin type or ethnicity-dependent differences in production of 25(OH)D, after accounting for baseline concentrations of 25(OH)D.145 Overall, a lack of research on photoprotection in people with different amounts of skin pigmentation hampers development of evidence-based advice regarding sun exposure.2.22 Exposure to high intensity solar radiation (such as on holidays) increases the concentration of 25(OH)D in serum with considerable concomitant DNA damage, while regular low dose exposure to UV radiation increases the concentration of 25(OH)D without accumulation of DNA damage
Synthesis of vitamin D in the skin, and subsequent increase in concentration of 25(OH)D3 in serum, is a marker of the benefit of exposure to UV-B radiation. The formation of cyclobutane pyrimidine dimers (CPDs) is a marker of risk and can be evaluated by measuring the concentration of T<>T dimers (a marker of DNA repair) in urine. Recent studies show that exposure to high doses of solar radiation, as experienced on beach holidays, increases concentrations of 25(OH)D in serum but with concomitant large increases in CPDs (Table 1).146 In contrast, a laboratory study involving people of different skin types showed that regular low-dose UV irradiation (less than half of the dose that would cause a minimal sunburn in a fair-skinned person (1.3 standard erythemal dose, SED, three times per week) led to increases in concentration of 25(OH)D3, with a plateau at 21 days. There were no detectable T<>T dimers in the urine (see Table 1).147 This suggests that public health messages should encourage regular low-dose sun exposure to improve vitamin D status, but avoidance of intermittent high-dose sun exposure.| Group | Location, latitude and duration (days) | N | Skin type (n) | Cumulative exposure, mean (SD) | % Body surface area exposed mean (SD) | Serum 25(OH)D (nmol L−1), mean (SD) | Urinary T<>T nmol, mean (SD) | Urinary T<>T fmol μmol−1 creatinine,d mean (SD) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | V | UV-B (kJ m−2)a | SEDb | Pre- | Post- | Pre- | Post- | Pre- | Post- | ||||
| a Dose of UV-B radiation multiplied by surface area of body exposed. b Standard erythemal dose, 1 SED = 100 J m−2 of erythemally-weighted exposure. c Erythemally weighted data from study files (unpublished). d Expressed as a function of creatinine concentration (unpublished study files) so that comparisons can be made with other published studies. e ND = not detectable. | ||||||||||||||||
| Danish holidaymakers146 | Tenerife 28°N, Canary Islands (6) | 25 | 0 | 11 | 11 | 3 | 0 | 6394 (3042) | 57.0c (24.7) | 50 (9.2) | 49.0 (23.3) | 70.5 (17.8) | 0.19 (0.2) | 3.8 (2.4) | 15.3 (19.1) | 293.6 (203.6) |
| Spanish holidaymakers146 | Tenerife 28°N, Canary Islands (6) | 20 | 0 | 6 | 9 | 5 | 0 | 3736 (1742) | 35.8c (12.7) | 44 (9.2) | 55.8 (23.1) | 72.4 (18.1) | 0.38 (0.4) | 2.1 (1.3) | 37.7 (44.1) | 194.3 (117.9) |
| Danish skiers146 | Wagrain 47°N, Austrian Alps (6) | 26 | 2 | 16 | 8 | 0 | 0 | 473 (164) | 50.6c (5.4) | 4 (2.6) | 50.6 (23.1) | 59.2 (20.0) | 0.10 (0.1) | 0.50 (0.8) | 8.3 (8.9) | 41.0 (61.8) |
| UK Caucasians147 | Manchester 53°N, UK (42) | 10 | 0 | 10 | 0 | 0 | 0 | 23.4 (0.0) | 35 (0.0) | 36.5 (13.0) | 54.3 (10.5) | NDe | ND | ND | ND | |
| UK South Asians147 | Manchester 53°N, UK (42) | 6 | 0 | 0 | 0 | 0 | 6 | 23.4 (0.0) | 35 (0.0) | 17.2 (6.3) | 25.5 (9.5) | ND | ND | ND | ND | |
2.23 Climate change and strategies for its mitigation will change UV-induced risks to human health
Skin cancer is now recognised in a number of countries as being an occupational health and safety issue.148 Outdoor workers such as agricultural workers may be particularly at risk from the combination of high levels of UV radiation and an increasing number of hot days, occurring as a result of global climate change.149 The most rapidly growing occupation in the USA is for technicians for renewable energy technologies, such as wind turbines.150 Several of the associated occupations are inherently outdoors, increasing risks of exposure to UV radiation.The direct effect of warming temperatures on genesis of skin cancer remains unclear. We have previously reported that animal and epidemiological studies suggest that higher temperatures may increase the risk of UV-induced skin carcinogenesis.151 In a recent large epidemiological study in the USA, the risk of BCC was lowest in the lowest temperature category for each of the quintiles of ambient UV radiation. However, the trend toward higher risk across the (increasing) temperature categories was not statistically significant.152 Not surprisingly, temperature and ambient UV radiation were highly correlated (r = 0.65). This correlation makes it difficult to assess independent or interactive effects of UV radiation and temperature in human studies. A recent study in hairless mice showed that animals that were exposed to a warmer environmental temperature (34 °C) prior to UV-B irradiation, compared to those not exposed to heat or exposed to heat following UV-B irradiation, had delayed and reduced tumour formation, and lower levels of UV-induced mutations.153 If there are similar effects in human skin, these results suggest that warmer temperatures are unlikely to increase the risk of UV-induced BCC.
3 Implications for terrestrial ecosystems in response to ozone depletion, ultraviolet radiation and interactive effects of rapid climate change
Recent research on the effects of current and future interactions of UV radiation and climate on terrestrial ecosystems is assessed. We also evaluate the way in which changing stratospheric ozone is driving climate in the Southern Hemisphere and the implications of this for ecosystems in terms of changes in wind patterns and strength as well as precipitation and drying conditions. Rapidly changing climatic conditions and associated changes in UV radiation extending to other regions may affect agriculture in several ways, reducing yield and the quality of some crops.Shifts in plant populations in some cases to higher elevations and higher latitudes are also being reported. These shifts may increase or decrease their exposure to UV radiation in novel environments producing positive and negative outcomes for acclimation of plants and conservation of plant species, their communities and habitats. UV radiation contributes to global warming through the breakdown of dead plant material, especially in dry areas, causing the release of carbon from terrestrial ecosystems as well as altering the availability of nutrients. Further progress has been made regarding the mechanisms underlying plant response to UV radiation, which aids our understanding of current and future consequences of the multiple interactive effects of climatic conditions and UV radiation.
Finally, in this section we report on some of the improved methodologies for measuring changes in UV radiation, important for increasing the accuracy and reliability of measurements.
3.1 Large ozone-driven changes in climate in the Southern Hemisphere have occurred over the past 3–4 decades and these climate changes continue to influence ecosystems in a variety of ways
Ozone depletion has influenced recent temperatures across Antarctica and been implicated in changes in precipitation patterns across the Southern Hemisphere and into Asia154–160 (Fig. 6; see also section 1.3). This has been linked to a highly positive phase of the Southern Annular Mode (SAM) or Antarctic oscillation (AAO),161 a mode of atmospheric variability that describes the north/south position of the westerly wind belt (i.e., jet stream) around Antarctica (see also ref. 162 and 163). A trend of the SAM towards its positive phase corresponds to a decrease in atmospheric pressure at high latitudes and to a contraction of the westerly wind belt towards Antarctica.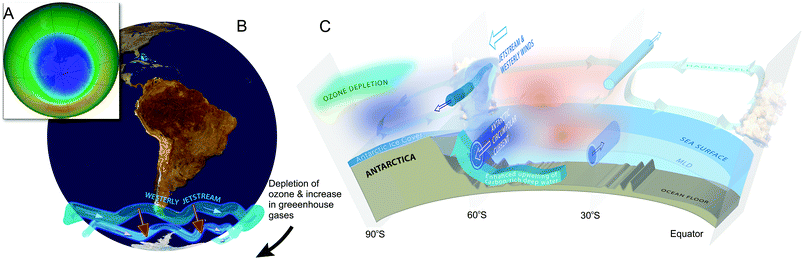 | ||
| Fig. 6 The Antarctic ozone ‘hole’ (A) and its impact on Southern Hemisphere atmospheric and oceanic circulation. Stratospheric ozone depletion and resultant cooling over Antarctica have pulled the polar jet stream towards the South (B). The speed of the jet has also increased (see ref. 162 for details). The polar shift in the jet and its increased strength have changed atmospheric and oceanic circulation throughout the Southern Hemisphere (B). These changes are manifest in a mode of variability called the Southern Annular Mode (SAM). The atmosphere can be envisioned as balancing on a seesaw that is shifting up and down between the polar latitudes (south of 60°S) and a latitude band between 40–55°S. The seesaw moves up and down with changes in mean sea level pressure (MSLP). As it pivots, the large cells that drive the winds and precipitation move towards or away from Antarctica. When MSLP around Antarctica falls, the westerlies are strong, and SAM is in its positive mode; when MSLP rises over those same regions, the westerlies weaken, and SAM is in its negative mode. Over the past century, increasing greenhouse gases and depletion of ozone have pushed the SAM towards its more positive phase (black arrow in B). The main effects of the ozone ‘hole’-induced positive phase of the SAM on the Southern Ocean are shown in C. The strengthening of the polar jet enhances the Antarctic Circumpolar Current and the associated overturning circulation (large blue-edged arrows). This drives increased upwelling of deep carbon-rich water and reduces the ability of the Southern Ocean to act as a sink for CO2.164 South of the polar jet stream, temperatures have decreased (blue), while to the North, temperatures have increased (red). The mean SAM index is now at its highest level for at least 1000 years.161 As a result, precipitation at high latitudes has increased and the mid-latitude dry-zone has moved south (see ref. 162 and 163). Clouds indicate areas with increased precipitation (over the equator and at the pole). (A. and B. were redrawn from ref. 165 and 162 with the ozone ‘hole’ over Antarctica in September 2017 reproduced from NASA Ozone Watch.166 C. was reproduced from ref. 162). | ||
Since the 1960s, warming and associated drying has resulted in an increase in frequency of forest fires, measured from fire scars of tree rings at mid- and high-latitudes on the west of the Andes.167 During the 2016–2017 fire season, more than 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 hectares burned in central and southern Chile (between ∼29°S and 40°S) driven by a long-lasting drought that was amplified by concurrent positive phases of SAM and ENSO conditions. Given the predicted continued positive phase of SAM (which is associated with ozone depletion as noted above and in section 1.3), increased wildfire activity in Southern South America is likely to continue through the 21st century.167 Decreased precipitation in this region also has negative implications for Chilean streamflow and the health of ecosystems as well as for production of hydroelectric power.168
000 hectares burned in central and southern Chile (between ∼29°S and 40°S) driven by a long-lasting drought that was amplified by concurrent positive phases of SAM and ENSO conditions. Given the predicted continued positive phase of SAM (which is associated with ozone depletion as noted above and in section 1.3), increased wildfire activity in Southern South America is likely to continue through the 21st century.167 Decreased precipitation in this region also has negative implications for Chilean streamflow and the health of ecosystems as well as for production of hydroelectric power.168
In contrast, the Eastern side of the Andes has experienced wetter conditions. For example, changes in fauna (ostracods and chironomids) from lake sediments in El Toro Lake (40°S, 70°W) indicate that the lake has become fresher (less saline) as a result of increased precipitation since the middle of the 20th century, associated with the positive phase of SAM.169 Ozone depletion and the positive phase of SAM are also associated with more extreme precipitation events in south-eastern South America (a very important area for food production11), and SW Madagascar.170 The rainfall of the southern Amazon basin has been reconstructed from Centrolobium microchaete tree rings171 and suggests that the extreme wet seasons (from droughts to extremely wet) since 1950 may be unmatched since 1799.
Along the Antarctic Peninsula and on nearby islands, increasing temperatures, consistent with ozone-depletion and increasing greenhouse gases,172 were associated with increased terrestrial productivity (microbial productivity, plant growth rates and carbon accumulation in moss beds) from the 1950s to the turn of the century.173 There is some evidence that these changes have reversed since 2000, consistent with the recent cooling of this region.173,174
In the sub-Antarctic islands, a positive phase of SAM is associated with better outcomes for some marine animals. A positive relationship between SAM and survival of juvenile wandering albatross has been found on the Crozet Islands.175 The authors speculate that this long-term climatic effect on recruitment age may be related to the progressive increase in weight observed in this species through the juvenile stages (see also ref. 176). Maternal condition in southern elephant seals on Macquarie Island varied by as much as 59 kg among years, with maternal mass positively associated with the SAM and negatively with sea ice extent.177 Similarly on the continent, modeling studies suggest that survival of juvenile emperor penguins is positively related to SAM, probably a result of the impacts of SAM on prey availability and sea ice extent (which determines the distance travelled to foraging areas178). These findings indicate pervasive and far-reaching effects of ozone-driven climate change on ecosystems across the Southern Hemisphere.179
3.2 Climate change alters seasonal weather patterns that then modify how UV radiation interacts with other environmental factors to influence crop ripening and stress tolerance
Understanding how plants respond to changes in UV radiation against a backdrop of other changing environmental factors is important for managing agricultural systems to maintain crop value and productivity under a changing climate. In certain cases, exposure to UV-B radiation can mitigate the adverse effects of environmental stress (e.g., drought).180,181 In other situations (e.g., supplemental UV-B radiation with increased tropospheric ozone181,182), UV-B radiation tends to accentuate the detrimental effects of concurrent stresses.Complex interactions between climate and UV radiation modify the timing of ripening of crops and the quality of harvest, with warmer temperatures and droughts changing the timing of ripening to coincide with the seasonal maximum for UV-B radiation.183 Drought and high UV-B radiation often co-occur, which can have positive effects on e.g., berry quality through changes in their sugar and antioxidant composition.184,185 In contrast, warmer temperatures may counteract the tendency for increased flavonoid accumulation with UV-B radiation.186
On the other hand, reducing the shade on fruit can increase their carotenoid, xanthophyll and flavonoid levels in some cases.187,188 However, flavonoids are generally induced by exposure to UV-A and UV-B radiation and ripening of fruits such as berry crops is hastened.188,189 The potential benefits are starting to be exploited by manipulating light conditions (via shading, canopy pruning or supplemental lighting) during growth and at the time of harvest.190,191 Based on their origin or provenances, certain crop and tree varieties or populations seem to be adapted to novel UV-B radiation and climate combinations. Investigators are now testing how well these crops actually perform under various future climate change scenarios.192
There continues to be significant uncertainty about how the combination of multiple environmental factors that change simultaneously, including UV radiation, are affecting food crops.
3.3 Plants are migrating to higher latitudes and elevations because of climate change and these shifts in geographic ranges present species with novel combinations of UV radiation and other environmental conditions
Establishment of certain plants at higher latitudes and elevations is occurring in response to climate change.193–195 These shifts in geographic ranges may alter the exposure of plants to UV-B radiation, since UV-B irradiances generally increase with increasing elevation and decrease with increasing latitude.20,196,197 In some cases, non-native, i.e., alien or introduced species, show higher migration potentials than native (indigenous) species.193,198 As the climate changes, a suite of other environmental conditions (e.g., diurnal and seasonal temperature patterns, moisture and nutrient availability, and associated pests, pathogens and competitors) co-occur with changes in exposure to UV radiation for migrating plants.195,199 At present, it is uncertain how the effects of these changes from exposure to UV radiation interact with unique combinations of effects of biotic and abiotic factors to influence performance of species and migration patterns. Whether native and non-native plants differ in their tolerance to UV-B radiation and acclimation to the changes is unclear.Plants that are native to high elevation environments (i.e., alpine) often show higher levels of UV-screening compounds (e.g., flavonoids) and other UV protective mechanisms than plants occurring at lower elevations.200–204 These differences are likely the result of the combined effects of changes in UV radiation with elevation, temperature, and other factors.205 For instance, low temperatures induce the production and accumulation of flavonoids, which increase screening from UV radiation and protect against oxidative stress.206–208 Populations of plants from high- and low-elevations may also differ in acclimation to changes in UV radiation.209 In wild potatoes (Solanum kurtzianum), populations grown at low elevation have relatively low constitutive (base-line) levels of leaf flavonoids but a high capacity for induction of flavonoids when UV radiation increases. In contrast, plants at high elevations have high base-line levels of flavonoids, but do not necessarily increase their UV-screening in response to supplemental UV-B radiation.210
A study examining UV-screening in plants growing in a tropical alpine environment with high UV radiation in Hawaii (Fig. 7A and B),211 found no differences in UV-screening between native and non-native species. In this study, production of UV-screening compounds increased with increasing elevation and UV-B radiation in a non-native species (Verbascum thapsus (mullein)) but did not vary with elevation in the native Vaccinium reticulatum (‘Ōhelo’). Whether these differences in acclimation of native and non-native species to changes in UV-B radiation are widespread is not yet known, although there are studies showing that non-native species acclimate better to environmental change than native species.212 This has consequences for diversity of plant species and composition of ecosystems.
 | ||
| Fig. 7 Plants growing in high elevation tropical alpine locations, such as Mauna Kea, Hawaii (A), experience some of the highest natural levels of solar UV radiation at the Earth's surface.213 These environments therefore provide excellent field sites for experiments designed to test the effects of extreme UV radiation conditions on plants (B). Shown here is an experiment using plastic film to reduce UV radiation to examine how these elevated levels of UV radiation influence plant growth and UV-screening. As plants migrate to higher elevations in response to climate change, they become exposed to higher levels of solar UV radiation as well as changes in several other abiotic and biotic factors. Understanding how plants will respond to UV radiation in the context of multiple environmental changes during migration is critical to assess how UV radiation and climate change will interact to modify the diversity and function of terrestrial ecosystems (Photographs by S. Flint). | ||
For plants expanding their distribution into higher latitudes, it is expected that they would experience less UV-B radiation that may then lead to a decline in UV-screening compounds, antioxidants and other metabolites involved in photo-protection.214 The cellular location of UV protective compounds in the same species can also show regional or latitudinal variation.208 A study of the same species of moss from Antarctica and Australia showed that the fast-growing temperate plants maintained high flavonoid concentrations within their cells, whereas the slow growing plants from Antarctica sequestered the same compounds in their cell walls. The latter may be a more beneficial location for the flavonoid compounds in plants experiencing frequent desiccation and freezing or it could be related to leaf longevity. Similar spatial variation in the distribution of UV screening compounds within plant tissue was observed between Arctic Vaccinium species with evergreen versus deciduous leaves.215
Ecosystems, and populations of plant species, including native species, have commonly responded over time to changing environmental conditions. However, with the recent rapid rate of climate change, increasing temperatures are of concern in terms of the conservation of species and habitats.179 Effects of temperature may, in turn, be amplified by the often-associated increased exposure to UV radiation at high elevations. Thus, migrations of plants to higher latitudes may result in decreased tolerance because of reduced exposures to UV radiation, as well as posing risks of disruption of species diversity and conservation of natural ecosystems and their services.
3.4 UV radiation and climate can have positive and negative effects on food quality and yield in agricultural systems
Extremes of temperature and low humidity may alter yields in some plants such as peas, while other changes, e.g., decreased plant growth and flowering, may be more influenced by UV radiation but without significant effects on pea pod production.216 Recent work confirms earlier findings that direct effects of UV radiation leading to modification of agricultural production occur through alterations in physiological and biochemical processes.185,217 With regard to direct effects, the prevalence and degree of severity of pathogen and pest attack on crops and other plants may be reduced by the biochemical reactions of the host plant, mediated through an increase in UV-induced polyphenolic compounds (see section 3.5).218,219The way in which food quality can be modified by UV radiation220–223 has implications for human health, since quality can be either positively or negatively affected by exposure to UV radiation and changing climate. Some of the potential health-promoting compounds that are enhanced by UV radiation and other environmental conditions include the polyphenolics, flavonoids and anthocyanins.224,225 These compounds, found in high amounts in certain fruits, vegetables and grains, have been suggested to afford protection against some diseases, e.g., coronary heart disease and type 2 diabetes, because of their free-radical scavenging capability (i.e., antioxidant activity).226–229
Response to UV radiation and other stressors is often cultivar- and genotype-dependent,208,223,225,230–232 findings that can be effectively exploited for specific crop quality outcomes in stressful environments. Many of these responses result in an increased accumulation of protective compounds such as the polyphenolics, which also are protective against plant pests.
UV-B radiation can increase production of seed oil, while decreasing protein, certain carbohydrates and fatty acids, depending on the amount of radiation. This was shown in a study on soybean seeds using realistic levels of biologically effective UV-B radiation (5–15 kJ m−2 day−1) in growth chambers with near ambient visible light.222 These changes have consequences for food quality. In addition, the effect of UV-B radiation may decrease the amounts of desirable monosaturated oleic acid, and increase the less desirable polysaturated linoleic and linolenic acids with implications for cardiac disease in humans. However, UV-B radiation also lowered the saturated palmitic fatty acid and stearic acid (the latter acid at higher levels of UV-B radiation) in the soybean seeds.222 These changes in important nutritional attributes of food need to be understood from the perspective of a rapidly changing climate together with potential interactive effects of different levels of UV radiation on the crops.
3.5 Agricultural intensification has increased during this century, resulting in increased planting densities and reduced row spacing, which can negatively affect quality and yield of crops by reducing plant exposure to the beneficial effects of solar UV radiation
Solar radiation, including the UV-B radiation component, is often a positive modulator of plant defenses against pathogens and pests. This beneficial role of solar radiation is sometimes caused by increased activity of hormonal pathways responsible for the activation of plant immunity (reviewed in ref. 233). In other cases, resistance is conferred by secondary metabolites that the plant accumulates in response to UV radiation, for example, phenolic compounds.218,233–235A common strategy to enhance biomass production and yield per unit area of many crops has been the implementation of management practices that increase light interception by the canopy, such as higher planting density, reduced row spacing, and fertilisation. These practices can reduce the exposure of individual plants to solar radiation and consequently its beneficial effects on defense responses, thereby making the crops more dependent on synthetic pesticides. Pests and diseases can account for a significant fraction (up to 25%) of pre-harvest crop losses in modern agricultural systems, and chemical controls are becoming increasingly regulated due to their potential negative environmental impacts. Manipulation of the molecular links between photoreceptors (see section 3.8) and plant defense responses, may help plant breeders to improve resistance to pests in agricultural and horticultural systems234,236–238
3.6 Exposure to solar radiation, including UV radiation, can accelerate the decomposition of plant litter by photochemical mineralisation and by facilitating the activity of microorganisms
The decomposition of dead plant material (i.e., litter) is a critical process controlling nutrient cycling and carbon storage in terrestrial ecosystems. Photodegradation occurs when UV radiation and short wavelengths of visible sunlight degrade lignin and other photo-reactive constituents of litter (i.e., photochemical mineralisation) and these changes then facilitate subsequent microbial decomposition. This latter aspect of photodegradation is often called ‘photo-priming’.239–241 Under some conditions, UV radiation can also retard decomposition by inhibiting the growth and activity of decomposer microorganisms (bacteria and fungi).240 The balance of these multiple mechanisms is determined by litter quality and environmental conditions that affect microbial activity, and will likely shift as the climate changes.Photodegradation is thought to be particularly important in arid and semi-arid ecosystems (i.e., drylands) where low moisture availability constrains the activity of decomposing microbes. However, photodegradation varies with species depending on leaf structure and plant chemistry (leaf mass/area, lignin and ratios of carbon![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nitrogen, C
nitrogen, C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N). Previous studies have demonstrated that the rate of photodegradation is positively associated with lignin levels.242 While some experimental and modeling studies fail to detect this relationship,243,244 this discrepancy likely reflects variation among species and litter type (e.g., leaf vs. woody litter) in the distribution of lignin with depth in plant tissues that determines the fraction of the total lignin content exposed to UV radiation. In addition, the litter position (standing litter vs. ground litter), stage of decomposition (i.e., early vs. late stages) and level of mixing of soil with litter will influence the degree of photodegradation245–247,248.
N). Previous studies have demonstrated that the rate of photodegradation is positively associated with lignin levels.242 While some experimental and modeling studies fail to detect this relationship,243,244 this discrepancy likely reflects variation among species and litter type (e.g., leaf vs. woody litter) in the distribution of lignin with depth in plant tissues that determines the fraction of the total lignin content exposed to UV radiation. In addition, the litter position (standing litter vs. ground litter), stage of decomposition (i.e., early vs. late stages) and level of mixing of soil with litter will influence the degree of photodegradation245–247,248.
Recent studies clarify how variation in environmental conditions and litter quality can modify the effects of UV radiation on photochemical mineralisation, photo-priming and microbial activity. For example, a field study conducted at several hyper-arid (annual precipitation <150 mm) locations in the Gurbantünggüt Desert, northern China, showed that solar UV radiation stimulated the decomposition of litter from all three plant species (grass and shrub) examined, and that the positive effect of UV radiation on decomposition increased with increasing precipitation in two of the three species.249 By comparison, a study conducted at two Mediterranean steppe locations (continental vs. maritime climates; annual precipitation = 248 vs. 362 mm, respectively) using grass and shrub litter, showed that UV radiation increased rates of decomposition in both species in the dry continental site but had no effect or a negative effect on decomposition at the high rainfall maritime site.250 Other studies251 further indicate that night-time moisture (humidity and dew) can influence the short-term, diel (daily) balance between day-time abiotic photodegradation and night-time microbial-driven decomposition in Mediterranean drylands. These findings suggest that in drylands the direct, abiotic effect of UV radiation on litter (i.e., photochemical mineralisation) dominates under the driest conditions, whereas the indirect, facilitative effect on microbial decomposition (photo-facilitation) tends to dominate under slightly moister conditions. However, when moisture levels and conditions are favourable to support elevated levels of microbial activity, UV radiation can have negative effects on decomposition, presumably because of direct inhibitory effects of the radiation on the decomposing microbes.
Whereas much of the research to date has focused on photodegradation in drylands, some studies indicate that this process can also occur in moist (e.g., forested) ecosystems.239 In forested ecosystems, the importance of photodegradation has been linked to canopy cover, levels of sunlight received by litter, and hence exposure to UV radiation.252 Shifts in vegetation type (e.g., grassland to shrubland, or loss of woody plant cover due to tree/shrub dieback) resulting from changes in land-use and climate change have the potential to alter the importance of photodegradation. This is due to the way in which litter is then exposed to UV radiation, e.g., increased shade because of encroachment of woody plants into grasslands and changes in soil-litter mixing. Also, alterations in litter chemistry resulting from changes in plant species composition (e.g., high C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N grass litter to low C
N grass litter to low C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N shrub/tree litter with the conversion of grasslands to desert shrublands) will affect the degree of photodegradation and microbial activity.253–255 These and other changes suggest that the role that UV radiation plays in regulating litter decomposition and carbon cycling in terrestrial ecosystems will likely change in the future as the ozone layer recovers and the climate continues to change.256,257
N shrub/tree litter with the conversion of grasslands to desert shrublands) will affect the degree of photodegradation and microbial activity.253–255 These and other changes suggest that the role that UV radiation plays in regulating litter decomposition and carbon cycling in terrestrial ecosystems will likely change in the future as the ozone layer recovers and the climate continues to change.256,257
3.7 UV radiation and other environmental factors, including climate change, are implicated in the production of several greenhouse gases by plants and plant communities
Methane (CH4) and nitrous oxide (N2O) are present in much lower atmospheric concentrations than carbon dioxide (CO2) but the global warming potentials of these gases are much greater than CO2 (28–36-fold for CH4 and 265–298-fold for N2O over 100 years195,258,259). Plants emit N2O and a small amount of CH4. Currently anthropogenic sources of CH4 exceed natural sources, while for N2O, natural sources are greater than anthropogenic.260Aerobic production of CH4 has been reported from a number of plant sources including leaf wax,261 and the cell wall compounds, cellulose, lignin262 and pectin.263 However, aerobic emissions of CH4 are small. For example, a modelling study estimates that just <0.2% of total global CH4 emissions come from pectin.264 A direct effect of UV-B radiation has been found on pectin. Other environmental factors such as water stress and warmer temperatures can modify UV-B-radiation-driven emissions of methane from certain plants in aerobic environments.265,266 A temperature of 28 °C compared with 22 °C, at ecologically-relevant UV-B radiation (5 kJ m−2 day−1 biologically effective UV radiation) under controlled conditions, resulted in higher emissions of CH4 from leaves, stems and roots of pea plants, with the highest emission from the stems (65.08 ± 4.12 ng (g dry mass)−1 h−1) and lowest for leaves (18.08 ± 0.96 ng (g dry mass)−1 h−1).265 Emission-enhancing interactive effects were found also for UV radiation and drought stress, and higher temperature and drought stress for different plant organs.265 Levels of emissions of CH4 are broadly consistent with previous studies (see ref. 267) but with large variations due to plant type, plant organ and environmental conditions.
Plant leaves and soil bacteria can produce N2O.268,269 Field experiments with filtered and unfiltered solar UV-B radiation showed that grasslands and their soil produced N2O in the dark, while solar UV radiation increased the N2O production.269 Precise data are difficult to obtain, since the N2O source from plant leaves is often augmented from natural and anthropogenic sources (e.g., from fertilisers) in addition to production by leaves. Calculations suggest that emissions of N2O from leaves may be 30% higher than previous estimates.269 This is important because these radiation-driven emissions are estimated to comprise between 7 and 24% of all natural production of N2O.260 Another compounding factor for N2O emissions, is the environmental feedback effect of increasing temperatures on the emissions from soil.270 Additional sources of N2O have also been reported from mosses and lichens,271,272 estimated to contribute up to 4–9% globally of the natural terrestrial N2O emissions.271,273
Changes in the composition of plant communities resulting from climate change and its interaction with UV radiation may also indirectly affect N2O and CH4 emissions at ecosystem scales. However, while anaerobic emissions of CH4 from peatlands and rice paddies are well researched,274 there have been few studies outside of wetlands.275,276 A study in a Holm oak (Quercus ilex) forest in Italy estimated CH4 emissions at the plant-community level based on parallel measurements of gas fluxes from the canopy and soil.277 This study found that the highest emissions (37.8 μmol m−2 h−1) coincided with solar noon on days when irradiance was highest in summer, but over the whole year the budget was approximately balanced (i.e., no net emission), switching between a CH4 source in summer and a sink in winter.
Estimating the contribution to the global CH4 budget by plants is complicated by many factors including large differences due to species and climate. Recent model calculations range between 1.2 to 24%.278–280 Calculations of N2O emissions from plants are also uncertain and vary in different studies depending on whether soil and plant contributions are quantified together or separately. N2O and CH4 are significant greenhouse gases and contribute to the dynamics of the stratospheric ozone layer. Thus, their sources and quantification are important in our understanding of interactions between UV radiation fluxes and climate change.
3.8 Recent progress in elucidating the molecular mechanisms that mediate plant perception of UV radiation can greatly increase our ability to manipulate responses to UV radiation in cultivated species
The functional characterisation of UV RESISTANCE LOCUS 8 (UVR8)281–283 represented a major step forward in UV photobiology, defining the first specific photoreceptor for UV-B radiation. Studies in recent years have endeavoured to establish the functional roles of the UVR8 protein photoreceptor in the control of plant acclimation to UV-B radiation.The central components of the UVR8 photocycle have been elucidated284,285 and the mechanism of action of UVR8 appears to be evolutionary conserved, from green algae to flowering plants.286–288 Many of the genes regulated by UVR8 are associated with protection against UV-B radiation and repair of damage by this radiation. Therefore, it is inferred that UVR8 plays a key role in protection against UV-B radiation.
Recent studies have focused on the hormonal signaling pathways that link UVR8 to physiological and biochemical functions in plants. These studies suggest that UVR8 modulates hormonal pathways involved in the regulation of growth (auxin and gibberellins) and defense responses (jasmonic acid).289–291 Based on these advances, it has been postulated that UVR8 is important for regulating plant growth and immunity in plant canopies,291 which might have important implications for agriculture and crop breeding (see sections 3.4 and 3.5).
Under solar radiation, the UVR8 protein occurs in equilibrium as a dimer or two-protein unit/monomer (dissociation into single protein units). This photo-equilibrium is regulated by UV-B radiation and is also influenced by temperature.292 Changes in the dimer/monomer ratio might be used by plants to “measure” UV-B radiation, although UVR8-independent signaling pathways, e.g., pathways activated by damage to DNA, are likely to play important roles in the perception of UV-B radiation under field conditions.293 Evidence obtained using genetically engineered lines of the model plant Arabidopsis thaliana with null mutations in the UVR8 locus, is helping to explain the roles of UVR8 in nature. Evidence from a limited number of studies suggests that uvr8 knock-out mutants are somewhat more sensitive to natural levels of solar radiation than wild-type plants294,295 and more sensitive to certain pathogens.296 However, additional information from field studies, measuring fitness components and using a broad array of experimental conditions and plant species, is needed to establish the adaptive importance of UVR8-mediated perception of UV-B radiation. Thus, more research is needed to link the mechanisms of action of the UV-B photoreceptor with plant function under field conditions.285,293
3.9 Improvements in methods and analytical techniques are reducing the uncertainty associated with reconstructions of solar radiation based on pollen biochemistry that track changes in past UV radiation over geological timescales
Reconstructions of past solar radiation using pollen from sediments and ice cores have the potential to help us better understand the evolution of the ozone layer and its interaction with climate change. Spores and pollen grains are coated with a biological polymer, sporopollenin, which is highly resistant to degradation over geological timescales and contains the phenolic compounds, para-coumaric acid and ferulic acid. The concentrations of these UV-absorbing compounds are considered to be proportional to the incident solar UV-B radiation flux received by the pollen.297–299 However, dose–response curves for the accumulation of these compounds are yet to be established, and we lack knowledge of the timing of this process during the production and release of pollen.163,300 Until the mechanism of this response is elucidated, and its relationship to incident UV-B and solar radiation established, the utility of this proxy for inferring historical changes in stratospheric ozone remains limited.301Seasonal environmental variability related to weather patterns and shade from plant canopies also affect incident solar UV-B radiation in ways that are difficult to retrospectively infer, making some reconstructions from proxies difficult to interpret. These potentially confounding factors may explain why changes in solar activity or ozone-depletion-related trends of UV-absorbing compounds in spores and pollen have not always been detected in the past.302 Methodological advances allowing faster and more precise processing of pollen samples are improving reconstructions.300,303 For instance, a reconstruction of incident solar radiation at Lake Bosumtwi in Ghana over a 140 thousand-year period, based on the phenolics from grass pollen contained in sediments, tracked known variations in the Earth's orbit.304 One potential strength of this proxy is that it can be interpreted independently of paleoecological and paleoclimatological records, in principle allowing solar radiation to be decoupled from climate or biotic changes.
3.10 Rapid advances in light-emitting diode technology is enabling more precise replication of the solar spectrum in controlled growth environments
The increasing availability of cost-effective light-emitting diodes (LEDs) is providing new opportunities to customise the light environment of plants grown commercially in greenhouses and controlled environments. The inclusion of UV-B and UV-A radiation in artificial lighting systems is now being considered as a means to improve food plant quality, crop vigour and plant defense.305 While high-power UV-A LEDs are currently available, so far UV-B LEDs have generally not proven useful in lighting systems because of their short life span and limited radiant output. However, new generation UV-B LEDs, including some with outputs over very narrow wavebands, exhibit greater stability and radiant power outputs.306,3073.11 Continuing refinement of protocols for the use of field-portable array spectrometers allows for better measurements of solar UV radiation in dynamic light environments with applications in plant science
Improved knowledge of the capacities and limitations of light-weight, field-portable array spectrometers has facilitated their wider use for recording dynamic variability in the light environment. These measurements may have wide utility but are particularly useful for measurements under clouds and plant canopies, and in artificial environments for plant growth, situations where the proportion of UV-B radiation to visible may be very different from that under clear skies. Those models of array spectrometer intended for measurement in both the visible and UV regions can capture this part of the solar spectrum with acceptable resolution for ecological studies provided that certain criteria are fulfilled.308,309 To obtain reliable measurements, these devices must be individually calibrated and specific protocols followed that minimise noise of the UV signal caused by stray visible light and limited dynamic range of the instruments.310,311 These spectrometers are particularly useful for validation of modelled radiative transfer through the canopy layers of forests and crop fields, although measurements in the early morning and evening when the sun is low in the sky should be avoided. Parallel applications for plant production are also envisaged whereby appropriate UV filters and UV-LEDs can be recommended for specific scientific purposes.3.12 The availability of small, lightweight, cost-effective UV-B dosimeters that can be placed on leaves may improve spatial resolution for measuring the UV radiation received by the leaves of plants in situ
These sensors will give researchers the opportunity to study the effects of fluctuating UV-B irradiances caused by variability in the micro-environment. For example, differences in plant architecture could result from changing angle-to-the-sun and canopy heterogeneity. Locating sensors on leaves would provide greater certainty in the estimation of doses as well as better characterisation of the environment through measurements at multiple locations. Two major handicaps to the adoption of dosimeters have been: (1) that they work for too short a time to be useful for ecological studies, and (2) they are insufficiently accurate to be reliable. Now, improved dosimeters are being developed with respect to their longevity312 and wavelength dependence,313 for use with plants as well as for materials and humans. One such new UV-B dosimeter, made from unstabilised polyvinyl chloride (PVC),314 complements an existing UV-A dosimeter manufactured using 8-methoxypsoralen.312 This presents an opportunity to compare in tandem UV-B and UV-A radiation doses received by leaves at different angles and exposures within a canopy. Such systems of multiple integrated dosimeters with sensitivity in different regions of the UV radiation regime have already been designed for use as ‘wearable tech’ on human skin (e.g., see ref. 315).4 Effects of ultraviolet radiation and climate change on aquatic ecosystems
Climate change is more important than stratospheric ozone depletion in regulating exposure of aquatic organisms to UV-B and UV-A radiation with important consequences for ecosystem services provided by inland and marine ecosystems. Here we highlight new insights into the effects of changes in exposure to solar UV-B and UV-A radiation related to ozone depletion and climate change on aquatic ecosystems, including the ability of organisms to adapt to these changes.While global changes in exposure to UV-B radiation are largely related to the depletion and more recent recovery from stratospheric ozone depletion in some regions (section 1), in aquatic ecosystems a variety of other factors including climate change are more important in controlling exposure of underwater organisms to UV-B and UV-A radiation. Some of these include changes in the concentrations of dissolved and particulate matter in aquatic ecosystems, the proximity and extent of runoff from terrestrial ecosystems, and the depth and mixing processes in inland, coastal, and open-ocean waters, all of which can compromise critical aquatic ecosystem services. Most aquatic organisms that have been tested show susceptibility to damage by solar UV radiation. However, solar UV radiation can also disinfect surface waters of pathogens, an ecosystem service that is being compromised by reduced UV transparency of some inland and coastal marine waters. Commercially produced UV-protective compounds that are used extensively as skin sunscreens, and in clothing and other industrial products, threaten the survival of corals and other aquatic organisms. Legislation to ban certain commercially-produced sunscreens is being considered in many places.
4.1 Climate change-induced increases in water temperature and associated increases in stratification generally intensify the negative effects of UV radiation in aquatic ecosystems
The adverse effects can, however, be reduced or amplified by the interactive effects of temperature, acidification, and nutrients. Increasing water temperatures associated with climate change shorten the duration of seasonal ice cover and lengthen seasonal exposure of aquatic ecosystems to UV radiation.316 It has been widely assumed that warming of the surface also results in shallower mixing317 potentially exposing organisms, especially free-floating ones (plankton), to higher UV radiation. However, observational evidence for this is lacking and recent analyses conclude that depth of mixing is equally (or more) affected by changes in wind strength and the interaction of currents318,319 (see also sections 4.7 and 5.7, Fig. 8).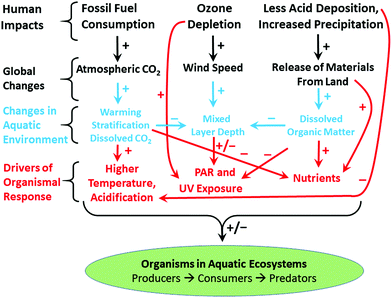 | ||
| Fig. 8 Diagram to illustrate the processes by which various human activities affect exposure to UV radiation and other related aspects of the structure and function of marine and freshwater ecosystems. Anthropogenic drivers are leading to a suite of global changes, which in turn alter aquatic ecosystems and their primary producers, consumers, and higher-level predators. While marine systems are generally becoming more acidic due to anthropogenic CO2 production, freshwater systems, on the other hand, are making extensive recoveries from previous anthropogenic acid deposition (acid rain) related to clean air act legislation in North America and Europe initiated in the early 1990s. However, this recovery from acidification is combining with increased precipitation to increase the concentrations of dissolved organic matter in some inland and coastal waters. Increased dissolved organic matter reduces the transparency of water to UV radiation (section 4.2), which, in turn, increases the survival of parasites and pathogens of humans and wildlife.320 | ||
The poleward shift of many ocean currents is increasing the temperature for some bottom-dwelling (benthic) organisms in environments such as those associated with coral reefs.321,322 Warmer temperatures and UV radiation interact to increase oxygen consumption and reduce rates of growth in some organisms such as intertidal fish323 and reduce the ability of corals to form calcium carbonate skeletons (through the process of calcification) that make up the structure of coral reefs. Acidification of the oceans impairs calcification in phytoplankton, reducing the thickness, and thus the UV protective effect, of the organism's outer shell (exoskeleton).324 Other effects of acidification may increase the sensitivity of larger attached algae (macroalgae)325 and certain animals to solar UV radiation. However, exposure to high levels of natural solar radiation stimulates calcification and thus increases protection against UV radiation in one species of phytoplankton investigated so far, offsetting the adverse impacts of acidification.326 Overall, interactions between increases in temperature and acidification of oceans with UV radiation can inhibit the growth, survival, or reproduction of some species, but benefit others, which has the potential to change the species composition of communities such as has been observed for macroalgae in the Arctic.327,328
In aquatic ecosystems, global change is also affecting nutrient availability, which is decreasing in some parts of the ocean due to warming-intensified stratification.329,330 However, nutrients are increasing in many lakes and coastal oceans due to transfer of particulate and dissolved organic matter and agricultural and other nutrients from terrestrial ecosystems (Fig. 8), which can also lead to anoxia and “dead zones” (http://www.noaa.gov/media-release/gulf-of-mexico-dead-zone-is-largest-ever-measured).320 Nutrients modify how solar UV radiation affects aquatic ecosystems and interacts with other stressors. For example, a series of laboratory experiments found that, when concentrations of nutrients were low and solar radiation was high, increased CO2 did not enhance, and UV radiation inhibited, growth of a common oceanic phytoplankton species, the diatom Thalassiosira weissflogii.331 However, when concentrations of nutrients were high, increased CO2 enhanced growth and sensitivity to UV inhibition of growth was reduced, most likely due to more efficient CO2 utilisation and enhanced repair.331 Laboratory experiments using artificial UV radiation and 400 nm cutoff filters and a freshwater zooplankton found that, at warmer temperatures, exposure to UV radiation lowered levels of a critical enzyme that takes up phosphorus into the body, thus limiting a nutrient necessary for zooplankton growth and reproduction.332 As a result of these effects on the lower levels of aquatic food webs, UV radiation and climate warming might interact to limit availability of food and reduce the abundance of recreationally and commercially important fish species.
4.2 A recent meta-analysis extends our previous knowledge of the adverse effects of UV-B radiation on aquatic organisms across trophic levels ranging from phytoplankton and zooplankton to amphibians and fish
Information on the relative tolerance to UV radiation of organisms at different trophic levels is essential if we are to understand the effects of solar UV radiation on aquatic ecosystems. Differential tolerance to UV radiation of primary producers, consumers, and top-predators has the potential to fundamentally alter trophic interactions and corresponding nutrient and energy flow as well as the community structure of aquatic ecosystems.A meta-analysis of UV-studies in more than 100 species of freshwater phytoplankton, zooplankton, fish and amphibians assessed the effects of UV-B radiation on survival, reproduction, development, growth, behaviour, metabolism, and molecular-cellular characteristics.333 Overall, UV-B radiation had adverse effects from the cellular to the population level on all organism groups. UV-B radiation affected survival and reproduction more than growth and development.333 No statistically significant or systematic differences in tolerance to UV-B radiation were observed among the major groups. However, for some characteristics, such as growth and survival, adverse effects of UV-B radiation on primary producers were greater than on consumers, such as zooplankton and fish.333 Other laboratory studies have shown that prior exposure of fish to UV-B radiation may confer benefits to offspring that reduce the adverse impacts of the radiation, but also increase the susceptibility of the offspring to disease infection.334
These freshwater studies included in the meta-analysis are consistent with the results of an earlier meta-analysis on marine organisms.335 Collectively these studies suggest no systematic differences in the effects of UV-B radiation on higher vs. lower trophic levels in freshwater or marine ecosystems. However, these analyses were mostly based on laboratory exposures rather than field studies and they did not consider spectral composition of the artificial UV radiation. Accounting for differences in the wavelength composition of artificial vs. natural solar UV radiation and the much stronger damaging effects of shorter wavelengths of UV radiation is essential to accurately assess the effects of UV radiation in nature. As assessments were for individual species, the meta-analysis did not examine community-level processes such as competition, predation, and succession. The lack of use of spectral data and the fact that laboratory studies, on average, report larger effects from UV-B radiation than field studies, emphasises the need for more realistic field studies to fully assess the overall effects of solar UV-B radiation on organisms at multiple trophic levels in aquatic ecosystems.
4.3 Increased input of coloured dissolved organic matter into aquatic systems reduces exposure to UV radiation and thus DNA-damage
Increased terrestrial input of coloured (chromophoric) dissolved organic matter (CDOM) into aquatic systems is a widespread phenomenon in many temperate, boreal and subarctic lakes, and is also projected to increase in Arctic permafrost areas as a result of climate change336–338 (see section 5.1). CDOM absorbs UV radiation, protecting organisms from direct damage to DNA and other targets of UV-B radiation.9,339 However, CDOM acts as a photosensitiser (S in reaction 1, Fig. 9) absorbing UV radiation (mainly UV-A) with the generation of various types of chemically reactive molecules (reactive oxygen species (ROS)). These ROS damage DNA and other cellular structures (C in reaction 2, Fig. 9). This has been demonstrated in recent laboratory studies on the effects of increased concentrations of CDOM where UV-induced production of ROS led to DNA damage (strand breaks), partially offsetting the benefit of UV-absorption provided to zooplankton.332,340 In contrast, field observations show that levels of DNA damage (strand breaks) in the larvae of an aquatic insect were 50 times lower in aquatic ecosystems with higher CDOM concentrations.341 This suggests that overall, CDOM absorption of UV radiation reduces DNA damage more than indirect chemical reactions increase the damage. However, all types of potential damage by UV radiation, not just DNA strand breaks, need to be considered to make any firm conclusions. Additional field observations and modeling research are required to improve our understanding of the balance between the protective vs. damaging effects of CDOM in natural systems and hence the net effect of UV radiation on the growth, reproduction, and survival of aquatic organisms.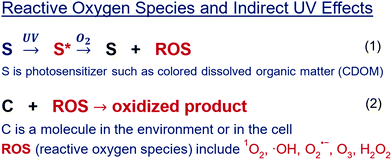 | ||
| Fig. 9 Reactive oxygen species (ROS) provide an important pathway for UV radiation to indirectly damage biological systems. Examples of ROS are singlet oxygen, hydroxyl radicals, superoxide radicals, ozone, and hydrogen peroxide (listed near the bottom of Fig. 9, respectively). Their induction by UV radiation occurs when a photosensitiser (S in reaction 1), absorbs UV radiation. Some of this absorbed energy puts the molecules into excited states (red colour in diagram), leading to reactions with oxygen molecules to produce ROS. These ROS can then oxidise molecules in the environment or in cells in living organisms (C. in reaction 2), hence damaging living cells and tissues and creating oxidised products. | ||
4.4 New models of vertical mixing have improved estimates of the inhibition by UV radiation of photosynthesis in Antarctic phytoplankton
These new models highlight the importance of properly representing vertical mixing in assessing effects of UV radiation in surface waters. Vertical mixing is an important process regulating exposure to UV radiation and responses of organisms in surface waters, and needs careful consideration especially for time-scales of tens of minutes to hours. This is illustrated in the time-dependent inhibition by UV radiation of photosynthesis in Antarctic phytoplankton.342 In this context, Smyth et al.343 modeled mixing and inhibition of photosynthesis by UV radiation in the Ross Sea Polynya, Antarctica. The focus of the study was vertical transport by Langmuir Circulation, a form of deep mixing created by the interaction of wind and waves in most open ocean (and lake) surface waters. The mixing effect resulted from photosynthesis being inhibited as simulated cells received exposure to UV radiation near the surface, as well as allowing for recovery from effects of UV radiation when transported to greater depth where they were exposed to moderate visible irradiance and no inhibitory UV radiation.Other, older approaches to modeling the mixing also resulted in reduced estimates of inhibition (relative to no mixing). However, the results differed from the more realistic Large Eddy Simulation by as much as 50% (range of modeled inhibition 4–13%). Thus, selection of the appropriate mixing model is an important consideration when estimating inhibition by UV radiation in Antarctic waters. The representation of mixing in surface waters will also be important in assessing the impact of other effects of UV radiation that vary at the critical time scale of minutes to hours, but mixing effects are less important in responses to UV radiation for processes happening over shorter or longer time periods.
4.5 The interaction of multiple types of environmental change because of human activities creates regional-specific changes in surface mixed layer depth and thus variable exposure to UV radiation of the ecosystem in the Southern Ocean
Climate change and ozone depletion are modifying prevailing wind patterns, freshwater inputs, and climate warming in oceans (see also sections 1.3, 3.1 and 4.1), with particularly strong effects in the Southern Ocean. These climate factors are expected to combine in regional-specific ways to affect the depth to which surface waters mix (see section 4.4). Shallower mixing enhances average exposure to UV radiation, while deeper mixing lowers the exposure but also lowers the light needed for photosynthesis (Fig. 8), which in the polar ocean can reach such a low level that phytoplankton populations are no longer sustained. In the warmest region of the Southern Ocean (to the north, in the Sub-Antarctic Zone) mixed layers are generally expected to become shallower due to warming and increased precipitation. Similarly, in the coldest region near the continent in the south, there will be shallower mixed layers due to warming and increased ice melt. In other regions, mixed layers are expected to deepen due to the increases in wind speed associated with poleward shifts of wind. These changes affect the southern part of the Sub-Antarctic Zone the most. The mechanism of the wind shift, related to ozone depletion, is discussed in section 1.3. The shifting wind patterns also have regional-specific effects within each zone. For example, there is an increase in flow of warmer air over the Antarctic Peninsula that is warming much more rapidly than other regions, while stronger winds are deepening the mixed layer in the eastern Indian Ocean and central Pacific Ocean.These changes in mixing of the ocean suggest that interactions between climate change and ozone depletion will create a mosaic of changing exposures to solar radiation that will vary throughout the Southern Ocean. The eventual recovery of stratospheric ozone may alter these trends. Changes in exposure as well as other concomitant environmental changes such as increased temperature, nutrient availability, and acidification all need to be considered in the selection of experimental conditions to assess organism and community response to Antarctic climate change, and thus the influence on ecosystem services provided by the world's oceans (see also section 4.1).
4.6 The ability of aquatic organisms to detect variations in exposure levels and behaviourally avoid solar UV radiation plays a key role in food chain interactions
The ability to perceive and respond behaviourally to UV radiation can markedly influence aquatic food webs. Zooplankton and fish that respond behaviourally to UV radiation have photoreceptors in the UV-A wavelengths. Since UV-A radiation penetrates deeper into waters than the shorter UV-B wavelengths, UV-A receptors in zooplankton provide environmental cues while simultaneously enabling avoidance of the water surface and excess exposure to damaging UV-B radiation. Behavioural responses to UV-A radiation can in turn influence the vertical distribution and thus foraging success of fish on zooplankton or other prey. Recent laboratory studies with mutant zebrafish that have far fewer UV-A-sensitive cones than normal zebrafish were used to investigate the importance of UV-A radiation in foraging.344 Non-mutant fish that sensed and responded to UV-A radiation exhibited a 24–90% greater foraging performance compared to the mutant fish. These results suggest that fish derive a considerable benefit from sensing and responding to UV-A radiation. However, in other laboratory experiments on coral reef fish, in which artificial UV radiation was manipulated by excluding wavelengths <400 nm,345 the presence of UV radiation reduced feeding rates and elevated respiration rates. The contrasting results of these two experiments may be due to different experimental approaches, or may point to the effects of UV radiation on fish foraging being species- or habitat-specific.Recent laboratory experiments have tested the behavioural response of zooplankton to artificial sources of UV radiation. Short-term (seconds) responses to UV-A radiation (from an LED source with a 375 nm peak), showed that some zooplankton with less pigmentation exhibited stronger avoidance of UV-A radiation than other species with more pigmentation.346 The short-term nature of these UV avoidance responses indicates that vertical distribution of zooplankton in lakes will be shallower with increases in sun angle, column ozone, cloud cover, or other factors that decrease UV irradiance or depth of penetration, such as presence of coloured dissolved organic matter or smoke from wildfires.339,347 This shallower distribution of zooplankton might increase their availability as food for fish and have important implications for the provision of valuable aquatic ecosystem services ranging from recreational and commercial fisheries to the role of zooplankton in helping clean the water through their feeding on algae and bacteria, including potential pathogens.
4.7 Commercial organic and nanoparticle UV-protective compounds frequently enter aquatic ecosystems and may pose substantial risks to aquatic organisms
Commercial UV-protective compounds are used extensively in sunscreens, plastics, adhesives, and industrial goods (e.g., clothing and construction materials) to protect against damage by UV radiation and adverse health effects (see sections 2 and 7). Some commercial organic and nanoparticle UV-protective compounds (e.g., oxybenzone and TiO2) reduce growth rates in phytoplankton,348,349 and cause developmental impairment in corals and sea urchins,350–352 as well as fish.353 These UV-protective compounds also affect the endocrine system and reduce feeding and energy reserves of invertebrates.354,355 A primary mode of toxicity for both organic and, to a lesser extent, nanoparticle UV-protective compounds, is via production of hydrogen peroxide (H2O2, Fig. 8).349 Production of H2O2 is greatly enhanced when these UV-protective compounds are exposed to solar UV radiation.349 Concentrations of commercial UV-protective compounds can be over 4-fold higher in wild fish compared with farmed fish, with the highest concentrations observed in fish near sewage outflows.356 Some commercial UV-absorbing compounds accumulate in organisms such as corals and may biomagnify in food webs.351,357 Because species have differential sensitivities to these commercial UV-absorbing compounds, these compounds may shift the relative composition of species in aquatic ecosystems.349 However, Cyperus alternifolius, a common wetland plant species, has the ability to metabolise oxybenzone, which suggests the possibility of remediation of waters contaminated by this compound.358Because of possible environmental impacts, the European Chemicals Agency has identified eight out of 16 of the most commonly used commercial UV-absorbing compounds in Europe as needing to be thoroughly evaluated over the next several years with consideration of possible future restrictions.359 The commercial development of natural alternatives to existing UV-absorbing compounds, such as mycosporine-like amino acids, may address the European Chemicals Agency concerns. Mycosporine-like amino acids have the advantage of being antioxidants as well as being effective at reducing direct damage from UV radiation.360
5 Interactive effects of solar ultraviolet radiation and climate change on biogeochemical cycles
Biogeochemical cycles involve the transport and transformation of materials within and between environmental compartments (i.e., land, water, atmosphere, and cryosphere). These cycles govern changes in the concentration and chemical form of nutrients and contaminants that affect organisms and ecosystems. For example, carbon and nitrogen cycles involve the transfer of energy between compartments as organic forms of these elements are consumed and transformed by biota. UV and visible solar radiation have substantial effects on biogeochemical cycles, which are also affected by changes in climate, land use, and air and water pollution. As a result, an understanding of biogeochemical cycles is essential for assessing the interactions between the effects of different components of global change, including changes in climate and stratospheric ozone. The following sections assess new findings regarding the cycling of carbon (sections 5.1–5.5), nitrogen (section 5.6), and organic contaminants (section 5.7).5.1 Warming of the Arctic and Antarctic is increasing ice-free habitats and thus exposure of organic matter to solar UV radiation
In the Arctic, human-caused warming leads to the loss of sea ice,361,362 reduction of ice and snow cover on land and in freshwaters, and thawing of permafrost soils.363 Increased exposure of organic matter to solar UV radiation in freshwaters,364,365 and in coastal and open ocean waters, leads to greater photochemical conversion of organic matter to greenhouse gases (carbon dioxide),366–368 nutrients (e.g., ammonium and nitrate), and altered organic matter. Organic matter that has been photochemically modified by UV radiation often supports more microbial respiration of carbon dioxide in both terrestrial and aquatic ecosystems compared to organic matter that has not been altered by UV radiation.369–371Specifically, Arctic sea ice is shrinking and thinning at a rate of 10% loss of area of sea ice per decade, with over 60% of total volume of ice lost in the last 30 years.361,362 The Arctic Ocean is predicted to be ice-free during the summer by the end of the century. Loss and thinning of sea-ice allows more UV radiation to reach the water surface, where terrestrially-derived organic matter is the main UV and light-absorbing constituent.369,372 Loss of sea ice cover increases exposure of terrestrially-derived organic matter to UV radiation in the Arctic Ocean and thus increases photochemical conversion of this organic matter to carbon dioxide. Uncertainties in these estimates include changes in cloud cover under ice-free scenarios.
Snowmelt on land and ice-off on lakes are occurring earlier in the spring,364,365 resulting in greater number of days in which UV radiation can reach land and freshwaters (on average by ca one day per year from 2000–2013). This earlier ice-off over freshwaters coincides with the period when the angle of the sun is the highest and thus, the UV radiation reaching the surface is most intense. This also coincides with the period when concentrations of terrestrially-derived organic matter, the main UV radiation-absorbing constituent,369 are at their highest. Thus, earlier ice-off on lakes in the spring and early summer in the Arctic can substantially increase photochemical conversion of this organic matter to carbon dioxide in freshwaters.369 Increased greenhouse gas emissions from freshwaters are important, because at present, carbon processing in freshwaters accounts for 40% of the net exchange of carbon between land and the atmosphere in the Arctic.373
There are at least 1500 billion tons of organic carbon (i.e., organic matter) in permafrost soils, almost twice as much carbon as currently exists in the atmosphere. Warming in the Arctic is causing permafrost soils to thaw, resulting in export of ancient organic matter to freshwaters and to the coastal ocean,374 where it is exposed to UV radiation.369 Based on current rates of warming in the Arctic, an estimated 130–160 billion tons of permafrost carbon could be available for conversion to carbon dioxide during this century.363 Currently, photochemical degradation of organic matter may account for ca. 30% of the carbon dioxide emitted from Arctic waters,369 and this estimate will likely increase as permafrost carbon comprises more and more of the organic matter exported from soils to sunlit surface waters of the Arctic.366,367,369 A substantial portion of this photochemical degradation of organic matter may be carried out by photochemically induced reactive oxygen species (ROS; see section 4.3 and Fig. 9).375
Like the Arctic, West Antarctica and the Antarctic peninsula are also warming, with West Antarctica one of the most rapidly warming locations on Earth.376 A recent projection concluded that warming may cause a 25% increase in total ice-free area on the Antarctic continent by the end of the century.377 Much of the increase in ice-free area is projected to be on the Antarctic Peninsula where reduction in ice cover will likely increase exposure to UV radiation of the underlying land surface (Fig. 10). The potential consequences to plants and animals remain highly uncertain.378 Loss of continental ice in Antarctica may shift the location of photochemical transformations of dissolved organic matter (DOM) from surface of glaciers and ice sheets379 to the coastal ocean.
 | ||
| Fig. 10 Actual and projected future ice-free area in the Antarctic continent. New Antarctic ice-free area (km2) predicted to emerge between 2014 and 2098 under climate forcing scenario RCP8.5 (Representative Concentration Pathway). Bar colours represent map locations of bioregions. Reprinted by permission from Macmillan Publishers Ltd.: J. R. Lee, B. Raymond, T. J. Bracegirdle, I. Chadès, R. A. Fuller, J. D. Shaw and A. Terauds, Nature, 2017, 547, 49–54, ©2017 (ref. 377). | ||
5.2 Exposure of plant litter to UV radiation and visible solar radiation accelerates carbon turnover in dryland ecosystems
Understanding the role of UV and visible solar radiation affecting carbon cycling in dryland ecosystems is important given that these ecosystems play a significant role as a control on the interannual variability in the global carbon balance.380 Direct effects of photodegradation (photochemical degradation of dead plant organic matter [plant litter] from exposure to solar radiation) have been linked to increased biotic turnover of organic compounds in plant litter in terrestrial ecosystems239,381,382 (see section 3.6). Direct photodegradation and photochemical changes in quality of litter (photo-facilitation), which increase biological degradation suggests that photodegradation can result in increases in emissions of CO2 to the atmosphere, particularly in semi-arid ecosystems.244 The implications of these recent studies suggest that photodegradation may have a larger impact on terrestrial carbon cycling than was previously thought. However, interactions with climate change, particularly changes in availability of water resulting from increased drought or increased rainfall may also determine the net effect of photodegradation on emissions of CO2 to the atmosphere. Direct photodegradation tends to increase under conditions of drought or extreme aridity.250,256,383 By comparison, photo-facilitation of biotic decomposition is enhanced under high moisture conditions, particularly at night.247,384 Given this important interplay between exposure to solar radiation and availability of water affecting decomposition of aboveground plant litter, future changes in UV radiation from stratospheric ozone recovery, and climate or land-use change could have important consequences for carbon cycling in dryland terrestrial ecosystems. Future studies are needed to identify under which conditions these variables may have antagonistic or synergistic effects on rates of decay of plant litter.5.3 The interactions between solar UV radiation and thermal stratification of lakes and oceans may reduce biological sequestration of atmospheric carbon dioxide
The biological carbon pump involves uptake of CO2 in photosynthesis by phytoplankton (primary production) and export of dead particulate organic matter to the sediment of lakes and oceans. Rates of primary production depend on various factors including the intensity of photosynthetically active radiation (PAR) in the mixed layer, and the abundance and availability of macro- and micro-nutrients, particularly dissolved inorganic nitrogen and iron.385,386 One factor that may result in limitation of nutrients is reduced upwelling of nutrients due to greater thermal stratification of lakes and oceans.387 This effect of thermal stratification could reduce carbon export from the surface ocean by 7–18% (range of scenarios from Earth system models).387 Furthermore, in some water bodies, stratification causes a decrease in the mixed layer depth (MLD), which, coupled with sunlight-induced decreases in UV-protective coloured dissolved organic matter, increases exposure of phytoplankton to solar UV radiation. However, surface warming of aquatic ecosystems due to climate change does not always increase stratification and result in shallower MLD.319,388 The MLD also depends on factors other than surface warming, particularly on wind speeds, with MLD increasing with stronger wind speeds. The strongest effect of thermal stratification of water bodies due to climate change is expected to be on limitation of nutrients. Regionally specific changes in MLD and hence in exposure to solar UV radiation may in addition influence uptake and fixation of CO2 by phytoplankton (see also sections 4.1 and 4.5).5.4 The balance between primary production and respiration in aquatic ecosystems may shift towards more respiration because of the interactive effects of solar UV radiation and climate change
This shift toward increased respiration would result in a decrease in the extent to which aquatic ecosystems are a sink for CO2. The balance between primary production by phytoplankton (autotrophs, i.e., producers) and respiration by bacterioplankton (mostly heterotrophs, i.e., consumers) is important in determining the sink strength of CO2 of aquatic ecosystems. In primary production, CO2 is taken up and oxygen (O2) is released, whereas in respiration, O2 is consumed and CO2 is released. A measure for the balance between primary production and respiration is net community production (NCP). Annually integrated rates of NCP vary from 0.5 to 15 mol of O2 per m2 per year in the sunlit zone of the global ocean.389 Hence, the global ocean is presently net autotrophic on average, i.e., the rate of primary production exceeds that of respiration. The combined effects of solar UV radiation and climate change could, however, shift some aquatic ecosystems towards net heterotrophy (non-producers) given that respiration increases with increasing temperature of aquatic ecosystems and with increasing concentrations of bioavailable DOM.389,390 In freshwater, coastal, and estuarine environments, an important source of DOM is terrestrially-derived dissolved organic matter (tDOM). The breakdown of tDOM to CO2 by bacterioplankton is slow. However, tDOM undergoes UV-induced transformations yielding labile organic carbon compounds that can then be metabolised to CO2 more efficiently by bacterioplankton.338,371 In addition, tDOM can also undergo direct photo-oxidation with release of CO2.366 Enhanced runoff of tDOM is expected because of thawing of permafrost,391 (also see section 5.1) and an increased frequency of extreme precipitation events, both associated with human-caused climate change392,393 (see also Fig. 8).Enhanced runoff of tDOM also increases the supply of nutrients to phytoplankton (see Fig. 8), which, in turn, would enhance fixation of CO2.394 The availability of nutrients to phytoplankton, particularly of the micronutrient, iron, is affected by solar UV radiation and by the interactions of solar UV radiation with other global changes such as acidification of aquatic ecosystems.395–397 Hence, the overall effect of solar UV radiation and global changes on the balance between primary production and respiration is an ongoing question but could shift towards net heterotrophy as a result of the combined effects of UV-induced formation of bioavailable carbon compounds from tDOM, and warming of aquatic ecosystems.
5.5 Thawing of permafrost is an important source of methane to the atmosphere where UV-induced reactions play a key role in controlling atmospheric concentrations of methane
Methane (CH4), a major greenhouse gas, causes about one-half of the warming (in terms of radiative forcing) of carbon dioxide (CO2). In addition to emissions of CH4 from human activities, particularly from agriculture, thawing of permafrost is an important source of CH4 to the atmosphere.398 The rate of emission of CH4 was found to be directly proportional to the rate of erosion of permafrost soil into lakes across the Arctic impacted by thermokarst failures (destabilised landscapes resulting from loss of permafrost).398 Hence, the emissions of CH4 from thawing permafrost soils can fuel a positive feedback process that further reinforces global warming and thawing of permafrost. In addition, wildfires are important causes of loss of permafrost in the northern boreal forest.399 The extent of loss of permafrost was shown to depend on the thickness of the insulating surface organic layer after a fire.399 Another important source of methane is direct production of methane as well as of carbon monoxide (CO) in wildfires.400 Carbon monoxide affects concentrations of CH4 in the atmosphere by scavenging the hydroxyl radical (˙OH) (see section 6.4). The occurrence of wildfires is increasing as a consequence of long-lasting droughts caused by climate change in some terrestrial ecosystems.339,392 A decline in concentrations of atmospheric ˙OH, due to various factors including stratospheric ozone recovery,1 and increased emissions of CO could potentially result in slower destruction of CH4 and thus amplify the positive feedback of CH4 to warming.5.6 Photochemical transformations of organic nitrogen in aquatic and terrestrial ecosystems may be altered by changes in solar UV radiation from ozone recovery, climate, or land-use change
When exposed to UV and visible solar radiation, organic nitrogen-containing compounds in aquatic ecosystems can be a source of ammonia and other forms of biologically-available nitrogen.401–404 In terrestrial ecosystems, modeling257 and empirical studies249,405 suggest that plant litter on the soil surface that is exposed to solar UV radiation may have increased microbial transformations (see also section 3.6) and leaching of nitrogen. Although the mechanisms responsible for transformation of nitrogen in aquatic and terrestrial ecosystems are poorly understood, it is likely that they involve coupled photochemical and microbiologically-mediated transformation of nitrogenous organic compounds. Global changes that reduce the amount of UV radiation could decrease production of inorganic nitrogen in aquatic and terrestrial ecosystems. However, other alterations from global changes, such as land-use or increased runoff of organic nitrogen from soils to aquatic ecosystems due to earlier snowmelt,406 may have the inverse effect, resulting in increased photo-production of inorganic nitrogen that consequently would be available for biotic uptake (see section 5.3).5.7 Human-induced changes in stratospheric ozone and runoff of UV-absorbing substances from land continue to alter photoreactions of contaminants that enter the environment
A wide range of commercially produced chemicals (e.g., contaminants) are intentionally or accidentally released into the environment by human activities. Solar radiation contributes to the degradation of many contaminants, and so plays a significant role in reducing their concentration in the environment. Photoreactions of contaminants occur through two general mechanisms. In the first mechanism (direct photoreaction), solar radiation directly absorbed by the contaminant results in changes in its chemical structure that can affect its persistence and fate in the environment. In the second mechanism (indirect photoreaction) the contaminant is transformed by reaction with short-lived reactive molecules that are produced through absorption of sunlight by substances such as coloured dissolved organic matter (CDOM). These processes usually include reactive oxygen species (see Fig. 9).The rates of both direct and indirect photoreactions are determined by how much UV radiation is absorbed. Photoreactions are, therefore, sensitive to changes in solar UV radiation due to changes in stratospheric ozone1 and/or other global changes (e.g., cloud cover or aerosols, see sections 1.6–1.8). Hence, recovery of stratospheric ozone1 could result in lower rates of photodegradation. In aquatic environments, increased runoff of rain and snow water caused by climate or land use changes (see sections 4.1 and 5.4) increases CDOM concentrations, which in turn can decrease photodegradation of contaminants by protecting them from exposure to UV radiation in deep freshwaters and coastal ocean (see section 4.3). However, in many aquatic environments, the rates of indirect photoreactions are proportional to concentrations of CDOM. Thus, decreases in rates of photodegradation of contaminants caused by protection from light by CDOM may be partially offset by increased rates of indirect photoreactions resulting from higher concentrations of CDOM. For example, this may occur near sewage treatment plants where indirect photoreactions can be accelerated due to the high concentration of CDOM in the effluent.407,408
Since our last update,9 additional research has confirmed that indirect photodegradation is an important mechanism for the breakdown of a wide-range of contaminants, including health-related compounds,409–411 carbon nanomaterials,412–415 pesticides,416–418 and UV filters.408 Improved evaluation of these photodegradation processes is now being achieved with readily accessible models of solar UV radiation320 to improve predictions of degradation of contaminants in the environment.416,419–421 These models can be further developed to include other environmental processes where photoreactions play a significant role e.g., the release of nanomaterials from commercial plastics (see sections 4.7 and 7.8) and the photoinactivation of disease-causing bacteria (see section 4.1). This would aid our ability to manage risks from contaminants in the environment.
6 Interactive effects of changing stratospheric ozone and climate on air quality and composition of the troposphere
Changes in stratospheric ozone and climate can influence the production and distribution of air pollutants such as ozone (O3), particulate matter (PM), nitrogen dioxide (NO2) and oxidised organic compounds. This occurs by several mechanisms: (i) modification of the transport of ozone from the stratosphere (stratosphere–troposphere exchange), (ii) variation of the intensity of UV-B radiation in the troposphere, inducing changes in key reactive compounds that degrade these air-pollutants, such as the hydroxyl radical (˙OH), and (iii) changes in meteorology, such as precipitation. In addition, changes in composition of the troposphere have resulted from actions taken under the Montreal Protocol to protect stratospheric O3, such as the use of replacements for ozone depleting substances (ODSs). These changes in air quality can impact human health directly and may also indirectly affect human health through compromising food security by damaging agricultural and natural ecosystems.6.1 Quantifying the amount of ozone transported from the stratosphere to the troposphere remains a significant challenge
The transport of air from the stratosphere brings significant amounts of ozone to the troposphere, contributing about 10% of the average background concentration.422 Quantifying this remains challenging, and an assessment of four alternative “best estimates” of atmospheric meteorology (combination of observations and meteorological models) for 1995–2011 found significant differences between the estimates of ozone transport to the troposphere, with differences in long-term variability as well as differences in the latitudes at which the bulk of the transport occurs.423 Ozone-sonde measurements of the variation of ozone with altitude have been used to estimate the amount of ozone transported to the troposphere at individual measurement sites in the Southern Hemisphere. This has then been combined with an atmospheric model to estimate the magnitude of the transport of O3 from the stratosphere to the troposphere in the Southern Hemisphere, but the estimates have fairly wide bounds (±20%).424This transport of O3 is known to be an important contributor to ambient (ground-level) O3 over large geographic scales. Future changes in stratospheric O3 and in climate would be expected to change the magnitude of this important process, but further work is needed to adequately quantify the amount of stratospheric O3 being transported to the troposphere and to estimate the implications for human and environmental health.
6.2 Better estimates of emissions have improved model predictions of ground-level ozone, although biases still exist
Prior atmospheric chemistry/transport models for the south-eastern USA had predicted concentrations of O3 much greater than observed.425 Based on detailed measurements of atmospheric composition, it was concluded that the emissions of nitrogen oxides (NOx) attributed to vehicular and industrial sources were too high (30–60%). Using the new data for emissions improved the agreement between the model and observations of ozone, but the model was still limited in its ability to reproduce the vertical variation of the concentration of O3 in the lowest km of the atmosphere.426 The development of mathematical models that can more accurately predict concentrations of O3 in the lower atmosphere is required to test the effectiveness of emission mitigation strategies and to accurately assess the role of UV-B radiation in the formation of O3.6.3 Concentration maxima for ambient ozone have shifted towards the equator in recent decades, changing the importance of global drivers such as climate change and stratospheric ozone
From 1980 to 2010, ambient ozone concentrations increased most noticeably in regions close to the equator. This shift is driven by changes in emissions of precursors of O3, such as anthropogenic volatile organic compounds (VOCs) and NOx,427 which are associated with developing economies. These precursors have increased in the tropical regions and decreased in temperate regions. Compared to mid-latitudes, these tropical regions experience higher UV-B radiation, temperature, and humidity; and ambient O3 will respond differently to stratospheric O3 and climate change. When assessing the global impact of stratospheric ozone depletion and climate on air quality into the future, this shift will need to be considered. Thus, in the future, more attention should be directed to characterising ambient O3 in tropical regions.6.4 Several approaches have been used to estimate the trend in the concentration of a key atmospheric oxidant (the hydroxyl radical) globally, with estimates ranging from constant to decreases by a factor of two in the last decade
Hydroxyl radicals (˙OH) are formed via UV-induced reactions involving the NOx–O3–OH cycle in the troposphere and they initiate the removal of methane and many other gases from the troposphere. Global concentrations of methane (CH4) have been increasing in the past two decades at varying rates, with a leveling off occurring during 1999–2006 (see Fig. 11 and section 5.5). The rate of change during this period was 0.6 ppb per year, while prior to 1999 rates were 6 ppb per year and from 2011–2016 they were 8.7 ppb per year.428 Several explanations for the leveling off have been proposed, mainly involving a reduction in emissions associated with fewer wetlands, and an increase in the rate of removal from the troposphere, i.e., because of an increase in the global concentrations of ˙OH, which change in response to factors such as increasing emissions of NOx. Year-to-year variations in ˙OH of around 1–5%, determined from global observations of other compounds that are destroyed by ˙OH, such as methyl chloroform, show some negative correlation with the trends in concentrations of CH4: an increase in concentrations of ˙OH during the CH4 hiatus,429 and decline after 2007.430 However, other analyses show that the hiatus could equally be explained by assuming constant concentrations of ˙OH and modulating CH4 emissions, or by a simultaneous reduction of ˙OH and CH4.431 | ||
| Fig. 11 Trends in global methane concentrations (Ed. Dlugokensky, https://www.esrl.noaa.gov/gmd/ccgg/trends_ch4/, downloaded 30th October 2017). | ||
It is also possible to estimate global or regional concentrations of ˙OH by other methods, e.g., from the variability of concentrations of hydrocarbons in the atmosphere,432 from long-term trends in concentrations of carbon monoxide,433 and from concentrations of organic nitrates in ice cores.434 However, because of the large uncertainties inherent in these methods, their use does not resolve the ambiguities implied by the trends in CH4. In summary, it is unclear whether concentrations of ˙OH really have changed substantially in the past few decades. Future changes in the lifetime and concentration of CH4 and many other compounds will depend on the extent to which the concentration of ˙OH is sensitive to changes in future environmental conditions including UV radiation, temperature, and humidity.
6.5 New research shows that the chemical composition of atmospheric particulate matter may alter its impact on human health
The health effects of particulate matter (PM) are currently evaluated mainly based on mass concentrations of particles smaller than 2.5 μm (PM2.5), without accounting for composition. Such particles are typically mixtures (see Fig. 12) of primary directly-emitted solid material such as soot, dust, metals and waxes combined with secondary compounds (e.g., oxygenated organics, sulfates, and nitrates). The concentration and chemical nature of these secondary compounds are modified by ˙OH and UV-B radiation. | ||
| Fig. 12 An Aerosol particle collected in the atmosphere of Mexico City. Soot spherules, shown in black, are about 20 nm in diameter, and are emitted directly from combustion sources. The (false) colours show a coating of sulfate (red) and oxygenated organics (blue), produced by UV-initiated, ˙OH-driven oxidation of sulphur dioxide and hydrocarbons (from ref. 435). | ||
Recent studies have attempted to determine the different health effects associated with specific chemical constituents of the particles. These studies take advantage of the observation that composition of particles vary by location and season and that these compositional changes might be associated with variations in adverse health effects. A literature review436 identified significant health effects associated with trace metals, mineral dust, inorganic water-soluble compounds, and organics, the two latter groups being composed in large part of secondary compounds (nitrates, sulfates, secondary organics). Ueda et al.437 showed that daily mortality in Nagoya (Japan) was marginally associated with elevated concentrations of sulfate, although there was also some association with nitrate, chloride, ammonium, potassium, elemental carbon (soot), and organic carbon. While more work is clearly needed, these early results confirm that particulate matter generated by UV radiation is a significant cause of health problems. Consideration of the chemical composition of atmospheric particulate matter should improve our ability to quantify the benefits of strategies for management of air quality.
6.6 Assessments of the health effects from air pollutants sensitive to changes in stratospheric ozone (e.g., tropospheric ozone, particulate matter, sulfate and NO2) have concluded that adverse effects occur following increases in short- or long-term exposures to concentrations of these pollutants
Exposure to outdoor air pollution and particulate matter has been classified by the International Agency for Research on Cancer (IARC) as a probable Group-1 human carcinogen and is recognised as an important driver of adverse effects on the health of humans.438Effects of changes in tropospheric O3 are most directly relevant to the purview of the Montreal Protocol, while there are interactions between UV-B radiation with NOx, SOx, and particulates (as mentioned above). Reports of associations between poor air quality and various diseases in humans continue to accumulate in the literature. Many of the recent studies on the effects of air pollutants report positive associations of several adverse health effects in addition to cancer, with increased levels of air pollution. This linkage between air pollutants and adverse health outcomes is thought to be mediated through direct effects on the cardiovascular and respiratory systems, the latter via oxidative stress and sustained inflammatory responses. Adverse responses in other internal organs are likely secondary to the direct effects on the circulatory and respiratory systems. The following provides an assessment based on several monographs, global studies, and meta-analyses that represent a small but comprehensive sample of the available recent literature.
A global analysis of 25-year trends (1990–2015) of the burden of disease attributable to ambient air pollution showed that PM2.5 was the fifth most important contributor to disease burden.439 It contributed to 7.6% of total global mortality in 2015. The authors estimated that long-term exposure to PM2.5 caused 4.2 million deaths and 103 million lost years of healthy life each year. Exposure to O3 caused an additional 254![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 deaths and 4.1 million lost years of healthy life, primarily due to chronic obstructive pulmonary disease. The global distribution of this estimate of the health impact is shown in Fig. 13. An economic assessment of health effects of PM2.5 in 2006 in China reported costs of USD151–177 billion.440 Of this, 90% was attributable to mortality estimated to be 1.7–2 million from all causes.
000 deaths and 4.1 million lost years of healthy life, primarily due to chronic obstructive pulmonary disease. The global distribution of this estimate of the health impact is shown in Fig. 13. An economic assessment of health effects of PM2.5 in 2006 in China reported costs of USD151–177 billion.440 Of this, 90% was attributable to mortality estimated to be 1.7–2 million from all causes.
6.7 Adverse effects of poor air quality on crop plants continue to be documented, especially in less-developed countries
The effects of air pollutants in the lower atmosphere, in particular O3, have been assessed in previous reports9 and these effects continue to be documented. Particulates, such as PM10 and PM2.5, have negligible effect on plants, while O3 is directly toxic. An economic assessment of the effects of O3 on production of wheat, rice, maize, and soybean in China, indicated annual losses of the order of USD3.4 billion.440Losses in these crops for 2006 were estimated at 9, 4.6, 0.44, and 0.34 million tonnes, respectively. Based on models of concentrations of O3 and its precursors NOx and volatile organic compounds (VOCs) in India, losses of yields of wheat and rice from O3 damage in the major production regions were estimated at 2.2 million tonnes (3.3%) and 2.05 million tonnes (2.5%), respectively.441 A similar study published in 2017 provided greater estimated damage from O3 in wheat and rice in India.442 These authors estimated that the total annual loss of yield in India as a whole was 4.0–14.2 million tonnes (4.2–15.0%) for wheat and 0.3–6.7 million tonnes (0.3–6.3%) for rice. Also in India, a study on several varieties of potatoes showed that concentrations of O3 above the accumulated exposure over the threshold of 40 ppb (AOT40) resulted in losses of 4.5–25%, depending on cultivar.443 Concentrations of O3 in the troposphere in most regions of Europe have decreased since the 1980s.444 Results of modeling indicate that future concentrations of O3 in the troposphere in Europe will likely continue to decrease in most regions to below the target of AOT40 by 2020. However, using the more sensitive metric of Phytotoxic Ozone Dose (POD, based on the accumulated ozone flux into leaves and needles), exceedances of toxic doses might persist in some European forestry regions into the 2050s. Continued monitoring and a better understanding of the processes that result in damage to crops will aid in developing strategies for management or mitigation.
6.8 Trifluoroacetic acid, which is produced through the breakdown of some fluorinated replacements for ozone depleting substances, remains an issue of concern in the atmosphere
The fate and effects of trifluoroacetic acid (TFA), the terminal breakdown product of several current-use refrigerants, were discussed in previous reports.9,445 Further information on effects of TFA in humans or the environment has not been published since the last Update Report;9 however, several papers have reported information about the fate and formation of TFA in the environment.Further experimental evidence has confirmed the recalcitrance of TFA to degradation in the environment. TFA was found to be stable to polychromatic radiation (200 to 1000 nm) for up to 16 h in laboratory tests.446 In the atmosphere, most TFA was measured in the vapour phase with less than 5% adsorbed to particles.447 The process of adsorption of TFA to soot particles was physical but is expected to be reversible by dissolution in water. A recent paper448 confirmed that TFA could be formed from several pharmaceuticals when they were exposed to conditions like those in waste-water treatment plants. TFA is also formed from several pesticides and, because of this, is subjected to the legislative criterion in the EU that pesticides (and metabolites) cannot exceed a concentration of 0.1 μg L−1 in drinking water.449 In some locations in the EU, concentrations of TFA exceed this criterion. This results in a dilemma in that the source of most of this TFA is likely not from pesticides; however, it is impossible to apportion the measured concentrations to pesticidal and non-pesticidal origins. Although TFA has been observed in surface- and drinking-water in Europe and other regions, it remains at concentrations that are well below those of toxicological concern.
7 Interactive effects of solar ultraviolet radiation and climate change on damage to materials
Solar UV radiation adversely affects the properties of materials used in outdoor construction, such as plastics and wood. The service lifetimes of these materials are influenced by the rates of degradation by solar UV radiation as well as other climate factors, especially temperature. Therefore, any change in the stratospheric ozone layer together with other climate factors determines their outdoor service lifetimes. However, several technologies including the use of UV stabilisers and surface treatments or coatings have been developed to mitigate these adverse effects. While these technologies can address any realistic UV radiation and climate scenario, they add to the cost of the material.The focus of this section is on recent advances in understanding of the mechanisms of UV radiation-induced degradation in materials, and on assessing the emerging technologies for stabilisation of materials against degradation mediated by UV radiation and climate.
7.1 Naturally-derived UV stabilisers for wood and plastic materials are being developed from wood extractives
Extractives are coloured secondary metabolites present in wood or wood bark that can be extracted with an organic solvent. Antioxidant compounds, particularly the catechol flavonoids, present in extractives from Acacia confusa act via multiple mechanisms to photostabilise sapwood of Cunninghamia lanceolata.450 In laboratory exposures, the extractives were as effective in controlling discolouration of wood due to lignin breakdown as synthetic UV stabilisers. Tannins (proanthocyanidins) extracted from bark of Pinus radiata also show similar stabilising effects.451,452 Incorporating either native or modified tannins at typical additive levels of <0.5% in acrylic coatings controlled the discolouration of timber in outdoor weathering studies, again out-performing conventional synthetic UV stabilisers at comparable concentrations. Given the move towards increased sustainability in the wood industry, these natural compounds show promise as alternatives to synthetic UV stabilisers.7.2 Wood-derived lignins or their depolymerised products can be used as a UV and oxidation stabiliser in commodity plastics
Cellulose and lignin are some of the most abundantly available forms of biomass on Earth. Lignins are thermally stable and can be readily processed with common plastics.453 They contain phenolic and other antioxidant functionalities454 that impart stability to the plastic against damage by UV radiation. At 2% (w/w), lignin imparted very good UV stability to polypropylene (PP) via a free-radical scavenging mechanism.455 At similar concentrations, Kraft-processed lignin (whereby lignin, which binds the cellulose fibres, is dissolved) from Eucalyptus sp. was reported to perform even better than a conventional hindered-amine light (HAL) stabiliser as the primary UV stabiliser in PP.456 De-polymerised products of lignin are also effective UV stabilisers in polyethylene and PP.457 This study on the effect of exposure to simulated solar UV radiation on the stiffness of PP found 2.5 wt% depolymerised Kraft lignin to be as effective as 0.5% (w/w) conventional stabiliser. Lignins, a by-product of the paper industry, can be a cost-effective and environmentally sustainable class of UV stabilisers for commodity plastics.7.3 The use of nano-powders as a protective surface coating is a promising approach to stabilise wood used outdoors and that is exposed to solar UV radiation
Wood surfaces exposed to solar UV radiation routinely need to be protected from discolouration and other damage.458 Surface protection of wood with coatings that contain fillers is a popular strategy to control discolouration of wood exposed outdoors.When used in lesser amounts, nanoscale oxides that absorb solar UV radiation can efficiently shield the wood surface from exposure to UV radiation. The effect of treating wood surfaces directly with nanoscale zinc oxide or titanium dioxide459,460 or with coatings carrying 1–3% (w/w) of these nanofillers461 was recently studied. These treatments reduced the discolouration of wood species by 48–57% in exposures to natural and/or simulated solar UV radiation, respectively. Arrays of zinc oxide nanorods (typically <100 nm in length), generated directly on the surface of wood, were particularly effective absorbers of UV radiation, resulting in less than 20% of the discolouration compared to that for untreated wood exposed to simulated solar UV radiation under laboratory-accelerated conditions.462
7.4 A new model predicting the degradation of wood by exposure to solar UV radiation also considers the effects of moisture content and the temperature of exposure
Rates at which wood discolours outdoors depends on the dose of solar UV radiation received as well as the ambient temperature.463 Most published studies only report the rates of discolouration relative to the dose of solar UV radiation. Degradation of thin samples of spruce wood weathered under outdoor- and laboratory-accelerated conditions has been studied using near infrared hyperspectral imaging.464 The regression models for lignin damage under UV exposure also included temperature and humidity as additional experimental variables.464 This is a first step towards a predictive model that will help estimate damage to timber exposed to solar UV radiation under different climatic conditions, although the model needs further validation.7.5 Nanofillers are effective for controlling degradation by solar UV radiation of some plastics, but may accelerate degradation in others
Reinforcing fillers are commonly used in plastics to increase their mechanical strength.465 However, some of these fillers may act as UV stabilisers as well. Because of their high surface area per unit mass, nanoscale UV-absorbing fillers are particularly efficient UV stabilisers in some plastics.466,467 For instance, incorporating 1% (w/w) nanoclay468,469 or rutile titanium dioxide (TiO2)470 in poly(lactic acid) plastics or 2% (w/w) TiO2 in acrylic films471 resulted in marked improvement in their UV stability in terms of loss of tensile properties467,469,470 or as weight loss.471 However, mixing 2% (w/w) of nanoclay in polystyrene472 or 5–8% TiO2 nanotubes in PP473 unexpectedly accelerated their photodegradation. The efficacy of nanofillers as UV stabilisers appears to vary with the plastic-filler combination and the amount of filler used. Further studies into their mechanisms of action is required prior to broader application of nanofillers as stabilisers in plastics.7.6 The release rates of carbon nanotubes through degradation by solar UV radiation of polycaprolactone nanocomposites are lower than expected based on their composition, thus reducing environmental and human health hazards
With an estimated production of 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 tonnes in 2016, carbon nanotubes (CNT) are being used to reinforce plastic products for numerous applications. Carbon nanotubes have a diameter of the order of tens of nm and lengths in the 1000s of nm. Upon weathering, these composites release CNTs (or microplastics containing CNTs) into the environment,474 posing a potential public health hazard474,475 (see also section 4.7). Mixing up to 20% (w/w) of CNT fillers in polyolefin plastics increases their strength modulus476 and electrical conductivity.477 Polycaprolactone nanocomposites (with CNTs) underwent substantial degradation under accelerated laboratory exposure to UV radiation. However, these composites released less CNTs from the weathered surface than expected based on their composition. During degradation, a mat of CNT gradually formed on the surface, preventing further release of CNTs or microplastics from the surface undergoing weathering.478 Nano- or microplastic released from weathering of this class of nanocomposites might be less of an environmental concern than expected. Whether or not this phenomenon generally holds for other CNT nanocomposites is not known.
800 tonnes in 2016, carbon nanotubes (CNT) are being used to reinforce plastic products for numerous applications. Carbon nanotubes have a diameter of the order of tens of nm and lengths in the 1000s of nm. Upon weathering, these composites release CNTs (or microplastics containing CNTs) into the environment,474 posing a potential public health hazard474,475 (see also section 4.7). Mixing up to 20% (w/w) of CNT fillers in polyolefin plastics increases their strength modulus476 and electrical conductivity.477 Polycaprolactone nanocomposites (with CNTs) underwent substantial degradation under accelerated laboratory exposure to UV radiation. However, these composites released less CNTs from the weathered surface than expected based on their composition. During degradation, a mat of CNT gradually formed on the surface, preventing further release of CNTs or microplastics from the surface undergoing weathering.478 Nano- or microplastic released from weathering of this class of nanocomposites might be less of an environmental concern than expected. Whether or not this phenomenon generally holds for other CNT nanocomposites is not known.
7.7 Self-healing polymers can contribute towards protecting the plastic components in solar photovoltaic panels against solar UV-induced degradation
Damage to the active layers of photovoltaic (PV) panels by solar UV radiation479,480 through micro-cracking and discolouration of components of the panel (especially the encapsulant,481,482 backing-sheet,483 and adhesive)481,483,484 limits their service lifetimes making them uneconomical for extended use.485Among the novel strategies researched to reduce their degradation under UV radiation, a new class of self-healing polymers effectively addresses the cracking of encapsulants in solar cells.486 Power conversion efficiency of the treated units can be maintained with only a ca. 15% loss over a 20-day period of laboratory accelerated exposure, compared to a 90% loss in an untreated device during the same period. A second promising approach includes the use of inorganic phosphor additives as UV radiation absorbers to control the degradation of plastic components.487 These enhancements contribute to increased service lives of solar PV devices.
7.8 Exposure of plastic litter to UV radiation during weathering is speculated to be responsible for generating secondary microplastics in the marine environment
Experimental evidence of this process of generating secondary microplastics has been reported for the first time.488,489 Occurrence of microplastics (particles < 5 μm) in the ocean environment is widespread and is an emerging environmental concern. Secondary microplastics are fragments of larger pieces of plastic debris generated due to exposure by solar UV radiation and the abrasion of weathered plastic litter with sand or water.488,489 The adverse impact of microplastics on marine ecosystems has been reported in several studies490 (and see section 4.7). Experimental studies now provide evidence that micro- and nano-plastics (in the size range of 30 nm to 60 μm) are generated when plastics in water are irradiated with UV radiation (320 to 400 nm) at 30 °C (ref. 491) under laboratory-accelerated conditions. A study on high-density polyethylene (HDPE) plastics, where exposure to simulated solar UV radiation was followed by mild abrasion with sand, also yielded microplastic fragments; 14–17% of the sample was lost after 5–6 months of exposure to UV radiation that simulated coastal exposure conditions.492 The study also showed the rates of fragmentation for exposure in water to be slower than that for sand.These recent studies unambiguously demonstrate for the first time the generation of microplastics under marine exposure conditions, thus facilitating better future estimates of microplastic loads in the environment.
7.9 Nanoscale fillers continue to be successfully evaluated as UV stabilisers for textile fibres, although new bio-based dyes also show promise as effective UV stabilisers for fibres and fabrics
Inorganic nanoparticles such as nano-zinc oxide (nano-ZnO) and silica-coated ZnO493 in polyester or cerium-doped nano-ZnO in cotton fibres494 have been shown to provide good protection from UV radation. However, there is emerging interest in the use of dyes derived from plant sources as stabilisers, with some yielding as high a UV protection factor (UPF) as 50+ in natural fibres such as cotton.495 Where ZnO was used as a UV radiation protector, Aloe gel extract was added as a natural capping agent for better dispersion of the pigment. At 0.8% (w/w), the stabiliser improved the UPF values of linen fabric from 7 to 61.496 While more work is needed to assess their commercial potential, inexpensive, sustainable biodegradable plant dyes, which also act as UV absorbers, are likely to stimulate considerable market interest.Author contributions
All authors of this article have contributed equally.Conflicts of interest
The opinions expressed in this report are those of the authors alone. Richard Zepp notes that this Perspective article has been reviewed in accordance with the U.S. Environmental Protection Agency's (U.S. EPA) peer and administrative review policies and approved for publication. Mention of trade names or commercial products does not constitute an endorsement or recommendation for use by the U.S. EPA.Acknowledgements
The authors gratefully acknowledge the following:Generous contributions by UNEP/Ozone Secretariat and the World Meteorological Organization (WMO) for the convened author meeting. Generous support by UNEP for the following authors is also acknowledged: Pieter Aucamp, Amy Austin, Carlos Ballaré, Krishna Pandey, and Seyhan Yazar (also supported by the Australia National Health and Medical Research Council Early Career CJ Martin Fellowship). Support by the U.S. Global Change Research Program is gratefully acknowledged for the following: Anthony Andrady, Paul Barnes (also supported by the Loyola University J. H. Mullahy Endowment in Environmental Biology), Germar Bernhard (also supported by Biospherical Instruments Inc.), Janice Longstreth, Sasha Madronich, Craig Williamson (also supported by Miami University and Ohio Eminent Scholar funding), and Kevin Rose was supported by the National Science Foundation Macrosystems Biology and Early NEON Science grant EF 1638704. Matthew Robson was funded by the Department of Biosciences, University of Helsinki, Finland and by the Academy of Finland decision #304519; Donat Häder, by the Bundesministerium für Umwelt, Naturschutz und Reaktorsicherheit; Samuel Hylander, by the Swedish Environmental Protection Agency; Patrick Neale, in part by the Smithsonian Institution and US National Science Foundation Grant DEB-1655622; Sten-Åke Wängberg, by the Swedish Agency for Marine and Water Management; Rose Cory, by NSF CAREER 1351745; and Anu Heikkilä, by the World Meteorological Organization, Global Atmosphere Watch; Richard Zepp, by the National Exposure Research Laboratory, Exposure Methods & Measurement Division, U.S. Environmental Protection Agency; Nigel Paul by the UK Department for Environment Food & Rural Affairs; Stephen Wilson, by the Centre for Atmospheric Chemistry, University of Wollongong (Australia); and Sharon Robinson, by a University of Wollongong Global Challenges Program. Alkiviadis Bais was supported by the Greek General Secretariat for Research and Technology; Richard McKenzie was funded by the New Zealand Government's Ministry for the Environment through the Ministry of Business, Innovation and Employment; Rachel Neale and Robyn Lucas receive salary funding from the National Health and Medical Research Council (Australia).
Erin Overholt is thanked for her assistance with the references. We thank Andrew Netherwood for designing and producing Fig. 6.
References
- M. P. Chipperfield, S. Bekki, S. Dhomse, N. R. P. Harris, B. Hassler, R. Hossaini, W. Steinbrecht, R. Thiéblemont and M. Weber, Detecting recovery of the stratospheric ozone layer, Nature, 2017, 549, 211–218 CrossRef CAS PubMed.
- J. Kuttippurath and P. J. Nair, The signs of Antarctic ozone hole recovery, Sci. Rep., 2017, 7, 585 CrossRef PubMed.
- L. Hu, S. A. Montzka, S. J. Lehman, D. S. Godwin, B. R. Miller, A. E. Andrews, K. Thoning, J. B. Miller, C. Sweeney, C. Siso, J. W. Elkins, B. D. Hall, D. J. Mondeel, D. Nance, T. Nehrkorn, M. Mountain, M. L. Fischer, S. C. Biraud, H. Chen and P. P. Tans, Considerable contribution of the Montreal Protocol to declining greenhouse gas emissions from the United States, Geophys. Res. Lett., 2017, 44, 8075–8083 CrossRef CAS.
- M. M. Hurwitz, E. L. Fleming, P. A. Newman, F. Li and Q. Liang, Early action on HFCs mitigates future atmospheric change, Environ. Res. Lett., 2016, 11, 114019 CrossRef.
- Y. Xu, D. Zaelke, G. J. M. Velders and V. Ramanathan, The role of HFCs in mitigating 21st century climate change, Atmos. Chem. Phys., 2013, 13, 6083–6089 CrossRef.
- S. Solomon, D. J. Ivy, D. Kinnison, M. J. Mills, R. R. Neely and A. Schmidt, Emergence of healing in the Antarctic ozone layer, Science, 2016, 353, 269–274 CrossRef CAS PubMed.
- D. J. Ivy, S. Solomon, D. Kinnison, M. J. Mills, A. Schmidt and R. R. Neely, The influence of the Calbuco eruption on the 2015 Antarctic ozone hole in a fully coupled chemistry-climate model, Geophys. Res. Lett., 2017, 44, 2556–2561 CAS.
- S. Solomon, D. Ivy, M. Gupta, J. Bandoro, B. Santer, Q. Fu, P. Lin, R. R. Garcia, D. Kinnison and M. Mills, Mirrored changes in Antarctic ozone and stratospheric temperature in the late 20th versus early 21st centuries, J. Geophys. Res.: Atmos., 2017, 122, 8940–8950 CAS.
- UNEP EEAP, Environmental effects of ozone depletion and its interactions with climate change: Progress report, 2016, Photochem. Photobiol. Sci., 2017, 16, 107–145 Search PubMed.
- S. Brönnimann, J.-C. Martín, R. Eugene, M. F. Andreas, M. Olaf, Z. Guang, A. Hideharu and Y. Yousuke, Tropical circulation and precipitation response to ozone depletion and recovery, Environ. Res. Lett., 2017, 12, 064011 CrossRef.
- Y. Wu and L. M. Polvani, Recent trends in extreme precipitation and temperature over southeastern South America: The dominant role of stratospheric ozone depletion in the CESM Large Ensemble, J. Clim., 2017, 30, 6433–6441 Search PubMed.
- D. J. Ivy, C. Hilgenbrink, D. Kinnison, R. A. Plumb, A. Sheshadri, S. Solomon and D. W. J. Thompson, Observed changes in the southern hemispheric circulation in May, J. Clim., 2016, 30, 527–536 Search PubMed.
- G. Chiodo, L. M. Polvani and M. Previdi, Large increase in incident shortwave radiation due to the ozone hole offset by high climatological albedo over Antarctica, J. Clim., 2017, 30, 4883–4890 CrossRef.
- G. L. Manney and Z. D. Lawrence, The major stratospheric final warming in 2016: dispersal of vortex air and termination of Arctic chemical ozone loss, Atmos. Chem. Phys., 2016, 16, 15371–15396 CAS.
- G. H. Bernhard, V. E. Fioletov, J.-U. Grooß, I. Ialongo, B. Johnsen, K. Lakkala, G. L. Manney and R. Müller, Ozone and UV radiation, in State of the Climate in 2016, Bull.Am. Meteorol. Soc., 2017, S154–S156 Search PubMed.
- D. J. Ivy, S. Solomon, N. Calvo and D. W. J. Thompson, Observed connections of Arctic stratospheric ozone extremes to Northern Hemisphere surface climate, Environ. Res. Lett., 2017, 12, 024004 CrossRef.
- F. Xie, J. Li, W. Tian, Q. Fu, F.-F. Jin, Y. Hu, J. Zhang, W. Wang, C. Sun, J. Feng, Y. Yang and R. Ding, A connection from Arctic stratospheric ozone to El Niño-Southern oscillation, Environ. Res. Lett., 2016, 11, 124026 CrossRef.
- J. G. Anderson, D. K. Weisenstein, K. P. Bowman, C. R. Homeyer, J. B. Smith, D. M. Wilmouth, D. S. Sayres, J. E. Klobas, S. S. Leroy, J. A. Dykema and S. C. Wofsy, Stratospheric ozone over the United States in summer linked to observations of convection and temperature via chlorine and bromine catalysis, Proc. Natl. Acad. Sci. U. S. A., 2017, 114, E4905–E4913 CrossRef CAS PubMed.
- J. Mok, N. A. Krotkov, A. Arola, O. Torres, H. Jethva, M. Andrade, G. Labow, T. F. Eck, Z. Li, R. R. Dickerson, G. L. Stenchikov, S. Osipov and X. Ren, Impacts of brown carbon from biomass burning on surface UV and ozone photochemistry in the Amazon Basin, Sci. Rep., 2016, 6, 36940 CrossRef CAS PubMed.
- A. F. Bais, R. L. McKenzie, G. Bernhard, P. J. Aucamp, M. Ilyas, S. Madronich and K. Tourpali, Ozone depletion and climate change: impacts on UV radiation, Photochem. Photobiol. Sci., 2015, 14, 19–52 CAS.
- S. Kazadzis, P. Raptis, N. Kouremeti, V. Amiridis, A. Arola, E. Gerasopoulos and G. L. Schuster, Aerosol absorption retrieval at ultraviolet wavelengths in a complex environment, Atmos. Meas. Tech., 2016, 9, 5997–6011 CrossRef CAS.
- T. Carlund, N. Kouremeti, S. Kazadzis and J. Gröbner, Aerosol optical depth determination in the UV using a four-channel precision filter radiometer, Atmos. Meas. Tech., 2017, 10, 905–923 CrossRef.
- M. Zhang, W. Gong, Y. Ma, L. Wang and Z. Chen, Transmission and division of total optical depth method: A universal calibration method for sun photometric measurements, Geophys. Res. Lett., 2016, 43, 2974–2980 CrossRef.
- R. McKenzie, B. Liley, M. Kotkamp and P. Disterhoft, Peak UV: Spectral contributions from cloud enhancements, AIP Conf. Proc., 2017, 1810, 110008 CrossRef.
- J. Badosa, J. Calbó, R. McKenzie, B. Liley, J.-A. González, B. Forgan and C. N. Long, Two methods for retrieving UV index for all cloud conditions from sky imager products or total SW radiation measurements, Photochem. Photobiol., 2014, 90, 941–951 CAS.
- J. Crawford, R. E. Shetter, B. Lefer, C. Cantrell, W. Junkermann, S. Madronich and J. Calvert, Cloud impacts on UV spectral actinic flux observed during the International Photolysis Frequency Measurement and Model Intercomparison (IPMMI), J. Geophys. Res.: Atmos., 2003, 108, D002731 Search PubMed.
- G. Pfister, R. L. McKenzie, J. B. Liley, A. Thomas and M. J. Uddstrom, Cloud climatology for New Zealand and implications for radiation fields, in UV Radiation and its Effects: An update, Royal Society of New Zealand, 2002, pp. 1–4 Search PubMed.
- M. Posyniak, A. Szkop, A. Pietruczuk, J. Podgórski and J. Krzyścin, The long-term (1964–2014) variability of aerosol optical thickness and its impact on solar irradiance based on the data taken at Belsk, Poland, Acta Geophys., 2016, 64, 1858–1874 Search PubMed.
- J. W. Krzyścin and P. Sobolewski, Trends in the surface UV radiation at the Polish Polar Station,Hornsund, Svalbard (77°00′ N, 15°33′ E), based on the homogenized time series of broad-band measurements (1996–2016) and reconstructed data (1983–1995), Atmos. Chem. Phys. Discuss., 2017, 2017, 1–17 Search PubMed.
- A. Sanchez-Lorenzo, A. Enriquez-Alonso, M. Wild, J. Trentmann, S. M. Vicente-Serrano, A. Sanchez-Romero, R. Posselt and M. Z. Hakuba, Trends in downward surface solar radiation from satellites and ground observations over Europe during 1983–2010, Remote Sens. Environ., 2017, 189, 108–117 CrossRef.
- H. Liu, B. Hu, L. Zhang, X. J. Zhao, K. Z. Shang, Y. S. Wang and J. Wang, Ultraviolet radiation over China: Spatial distribution and trends, Renewable Sustainable Energy Rev., 2017, 76, 1371–1383 CrossRef.
- N. A. Cabrol, U. Feister, D.-P. Häder, H. Piazena, E. A. Grin and A. Klein, Record solar UV irradiance in the tropical Andes, Front. Environ. Sci., 2014, 2(19) DOI:10.3389/fenvs.2014.00019.
- R. L. McKenzie, B. Liley and S. Madronich, Critical appraisal of data used to infer record UVI in the Tropical Andes, Photochem. Photobiol. Sci., 2017, 16, 785–794 CAS.
- T. VoPham, J. E. Hart, K. A. Bertrand, Z. Sun, R. M. Tamimi and F. Laden, Spatiotemporal exposure modeling of ambient erythemal ultraviolet radiation, Environ. Health, 2016, 15, 111 CrossRef PubMed.
- D. P. Igoe, A. Amar, A. V. Parisi and J. Turner, Characterisation of a smartphone image sensor response to direct solar 305 nm irradiation at high air masses, Sci. Total Environ., 2017, 587, 407–413 CrossRef PubMed.
- B. Mei, R. Li, W. Cheng, J. Yu and X. Cheng, Ultraviolet radiation measurement via smart devices, IEEE Internet of Things J., 2017, 4, 934–944 CrossRef.
- P. Padrik, A. Valter, E. Valter, A. Baburin and K. Innos, Trends in incidence and survival of cutaneous malignant melanoma in Estonia: a population-based study, Acta Oncol., 2017, 56, 52–58 CrossRef PubMed.
- A. M. Glazer, R. R. Winkelmann, A. S. Farberg and D. S. Rigel, Analysis of trends in US melanoma incidence and mortality, JAMA Dermatol., 2016, 153, 225–226 CrossRef PubMed.
- GBD 2016 Disease and Injury Incidence and Prevalence Collaborators, Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016, Lancet, 2017, 390, 1211–1259 CrossRef.
- E. Garnett, J. Townsend, B. Steele and M. Watson, Characteristics, rates, and trends of melanoma incidence among Hispanics in the USA, Cancer, Causes Control, 2016, 27, 647–659 CrossRef PubMed.
- B. A. Kohler, R. L. Sherman, N. Howlader, A. Jemal, A. B. Ryerson, K. A. Henry, F. P. Boscoe, K. A. Cronin, A. Lake, A. M. Noone, S. J. Henley, C. R. Eheman, R. N. Anderson and L. Penberthy, Annual report to the nation on the status of cancer, 1975–2011, featuring incidence of breast cancer subtypes by race/ethnicity, poverty, and state, J. Natl. Cancer Inst., 2015, 107, djv048 CrossRef PubMed.
- K. Mahendraraj, K. Sidhu, C. S. Lau, G. J. McRoy, R. S. Chamberlain and F. O. Smith, Malignant melanoma in African-Americans: A population-based clinical outcomes study involving 1106 African-American patients from the Surveillance, Epidemiology, and End Result (SEER) Database (1988–2011), Medicine, 2017, 96, e6258 CrossRef PubMed.
- E. de Vries, M. Sierra, M. Pineros, D. Loria and D. Forman, The burden of cutaneous melanoma and status of preventive measures in Central and South America, Cancer Epidemiol., 2016, 44(Suppl 1), 100–109 CrossRef PubMed.
- T. Tomizuka, K. Namikawa and T. Higashi, Characteristics of melanoma in Japan: a nationwide registry analysis 2011–2013, Melanoma Res., 2017, 27, 492–497 CrossRef PubMed.
- K. D. Bishop and A. J. Olszewski, Epidemiology and survival outcomes of ocular and mucosal melanomas: a population-based analysis, Int. J. Cancer, 2014, 134, 2961–2971 CrossRef CAS PubMed.
- P. T. Bradford, W. F. Anderson, M. P. Purdue, A. M. Goldstein and M. A. Tucker, Rising melanoma incidence rates of the trunk among younger women in the United States, Cancer Epidemiol., Biomarkers Prev., 2010, 19, 2401–2406 CrossRef PubMed.
- C. Naouali, M. Jones, I. Nabouli, M. Jerbi, H. Tounsi, M. Ben Rekaya, M. Ben Ahmed, B. Bouhaouala, O. Messaoud, A. Khaled, M. Zghal, S. Abdelhak, S. Boubaker and H. Yacoub-Youssef, Epidemiological trends and clinicopathological features of cutaneous melanoma in sporadic and xeroderma pigmentosum Tunisian patients, Int. J. Dermatol., 2017, 56, 40–48 CrossRef PubMed.
- T. M. Elliott, D. C. Whiteman, C. M. Olsen and L. G. Gordon, Estimated healthcare costs of melanoma in Australia over 3 years post-diagnosis, Appl. Health Econ. Health Policy, 2017, 15, 805–816 CrossRef PubMed.
- P. K. Goon, D. C. Greenberg, L. Igali and N. J. Levell, Squamous cell carcinoma of the skin has more than doubled over the last decade in the UK, Acta Derm.-Venereol., 2016, 96, 820–821 Search PubMed.
- P. K. C. Goon, D. C. Greenberg, L. Igali and N. J. Levell, Predicted cases of UK skin squamous cell carcinoma and basal cell carcinoma in 2020 and 2025: horizon planning for National Health Service dermatology and dermatopathology, Br. J. Dermatol., 2017, 176, 1351–1353 CrossRef CAS PubMed.
- J. Rubio-Casadevall, A. M. Hernandez-Pujol, M. C. Ferreira-Santos, G. Morey-Esteve, L. Vilardell, G. Osca-Gelis, N. Vilar-Coromina and R. Marcos-Gragera, Trends in incidence and survival analysis in non-melanoma skin cancer from 1994 to 2012 in Girona, Spain: A population-based study, Cancer Epidemiol., 2016, 45, 6–10 CrossRef CAS PubMed.
- J. G. Muzic, A. R. Schmitt, A. C. Wright, D. T. Alniemi, A. S. Zubair, J. M. Olazagasti Lourido, I. M. Sosa Seda, A. L. Weaver and C. L. Baum, Incidence and trends of basal cell carcinoma and cutaneous squamous cell carcinoma: a population-based study in Olmsted County, Minnesota, 2000 to 2010, Mayo Clin. Proc., 2017, 92, 890–898 CrossRef PubMed.
- L. G. Gordon and D. Rowell, Health system costs of skin cancer and cost-effectiveness of skin cancer prevention and screening: a systematic review, Eur. J. Cancer Prev., 2015, 24, 141–149 CrossRef PubMed.
- A. Lomas, J. Leonardi-Bee and F. Bath-Hextall, A systematic review of worldwide incidence of nonmelanoma skin cancer, Br. J. Dermatol., 2012, 166, 1069–1080 CrossRef CAS PubMed.
- D. Schadendorf, C. Lebbe, A. Zur Hausen, M. F. Avril, S. Hariharan, M. Bharmal and J. C. Becker, Merkel cell carcinoma: epidemiology, prognosis, therapy and unmet medical needs, Eur. J. Cancer, 2017, 71, 53–69 CrossRef PubMed.
- K. York, N. C. Dlova, C. Y. Wright, N. P. Khumalo, P. E. Kellett, R. Kassanjee and A. Mosam, Primary cutaneous malignancies in the Northern Cape Province of South Africa: A retrospective histopathological review, S. Afr. Med. J., 2016, 107, 83–88 CrossRef CAS PubMed.
- N. A. Al-Dawsari and N. Amra, Pattern of skin cancer among Saudi patients attending a tertiary care center in Dhahran, Eastern Province of Saudi Arabia. A 20-year retrospective study, Int. J. Dermatol., 2016, 55, 1396–1401 CrossRef PubMed.
- A. Mittal and O. R. Colegio, Skin cancers in organ transplant recipients, Am. J. Transplant., 2017, 17, 2509–2530 CrossRef CAS PubMed.
- G. L. Garrett, S. E. Lowenstein, J. P. Singer, S. Y. He and S. T. Arron, Trends of skin cancer mortality after transplantation in the United States: 1987 to 2013, J. Am. Acad. Dermatol., 2016, 75, 106–112 CrossRef PubMed.
- C. C. Wang, C. H. Tang, C. Y. Wang, S. Y. Huang and Y. M. Sue, Risk of skin cancer in patients on chronic haemodialysis: a nationwide, population-based study in Taiwan, Br. J. Dermatol., 2016, 175, 1175–1182 CrossRef CAS PubMed.
- M. Hortlund, L. S. Arroyo Muhr, H. Storm, G. Engholm, J. Dillner and D. Bzhalava, Cancer risks after solid organ transplantation and after long-term dialysis, Int. J. Cancer, 2017, 140, 1091–1101 CrossRef CAS PubMed.
- Z. R. Chalmers, C. F. Connelly, D. Fabrizio, L. Gay, S. M. Ali, R. Ennis, A. Schrock, B. Campbell, A. Shlien, J. Chmielecki, F. Huang, Y. He, J. Sun, U. Tabori, M. Kennedy, D. S. Lieber, S. Roels, J. White, G. A. Otto, J. S. Ross, L. Garraway, V. A. Miller, P. J. Stephens and G. M. Frampton, Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutationalburden, Genome Med., 2017, 9, 34 CrossRef PubMed.
- I. Martincorena, A. Roshan, M. Gerstung, P. Ellis, P. Van Loo, S. McLaren, D. C. Wedge, A. Fullam, L. B. Alexandrov, J. M. Tubio, L. Stebbings, A. Menzies, S. Widaa, M. R. Stratton, P. H. Jones and P. J. Campbell, Tumor evolution. High burden and pervasive positive selection of somatic mutations in normal human skin, Science, 2015, 348, 880–886 CrossRef CAS PubMed.
- S. Brown, C. M. Pineda, T. Xin, J. Boucher, K. C. Suozzi, S. Park, C. Matte-Martone, D. G. Gonzalez, J. Rytlewski, S. Beronja and V. Greco, Correction of aberrant growth preserves tissue homeostasis, Nature, 2017, 548, 334–337 CrossRef CAS PubMed.
- A. Stang and K. H. Jockel, Does skin cancer screening save lives? A detailed analysis of mortality time trends in Schleswig-Holstein and Germany, Cancer, 2016, 122, 432–437 CrossRef PubMed.
- J. Hubner, A. Waldmann, A. C. Geller, M. A. Weinstock, N. Eisemann, M. Noftz, S. Bertram, S. Nolte, B. Volkmer, R. Greinert, E. Breitbart and A. Katalinic, Interval cancers after skin cancer screening: incidence, tumour characteristics and risk factors for cutaneous melanoma, Br. J. Cancer, 2017, 116, 253–259 CrossRef CAS PubMed.
- A. F. Duarte, B. Correia, A. Picoto, A. Costa Pereira, E. Nagore and O. Correia, Behaviour towards sun exposure, skin self-examination and skin cancer knowledge of educators, health professionals and the general population - cross-sectional study, J. Eur. Acad. Dermatol. Venereol., 2017, 31, e132–e135 CrossRef CAS PubMed.
- L. Pil, I. Hoorens, K. Vossaert, V. Kruse, I. Tromme, N. Speybroeck, L. Annemans and L. Brochez, Cost-effectiveness and budget effect analysis of a population-based skin cancer screening, JAMA Dermatol., 2017, 153, 147–153 CrossRef PubMed.
- W. A. Woo, C. D. Mitchell, E. Wakefield and J. Hextall, The importance of full skin examination in diagnosing cutaneous melanoma, Clin. Exp. Dermatol., 2017, 46, 674–675 CrossRef PubMed.
- U. S. Preventive Services Task Force, K. Bibbins-Domingo, D. C. Grossman, S. J. Curry, K. W. Davidson, M. Ebell, J. W. Epling Jr., F. A. Garcia, M. W. Gillman, A. R. Kemper, A. H. Krist, A. E. Kurth, C. S. Landefeld, C. M. Mangione, W. R. Phillips, M. G. Phipps, M. P. Pignone and A. L. Siu, Screening for skin cancer: US Preventive Services Task Force Recommendation Statement, J. Am. Med. Assoc., 2016, 316, 429–435 CrossRef PubMed.
- P. K. Sandhu, R. Elder, M. Patel, M. Saraiya, D. M. Holman, F. Perna, R. A. Smith, D. Buller, C. Sinclair, A. Reeder, J. Makin, B. McNoe, K. Glanz and F. Community Preventive Services Task, Community-wide interventions to prevent skin cancer: two community guide systematic reviews, Am. J. Prev. Med., 2016, 51, 531–539 CrossRef PubMed.
- S. Everett Jones and G. P. Guy, Jr., Sun safety practices among schools in the United States, JAMA Dermatol., 2017, 153, 391–397 CrossRef PubMed.
- G. P. Guy, Jr., Z. Berkowitz and M. Watson, Estimated cost of sunburn-associated visits to US hospital emergency departments, JAMA Dermatol., 2017, 153, 90–92 CrossRef PubMed.
- M. F. Taylor, D. Westbrook and P. Chang, Using UV photoaged photography to better understand Western Australian teenagers’ attitudes towards adopting sun-protective behaviors, Int. J. Adolesc. Med. Health, 2016, 28, 45–53 Search PubMed.
- A. H. Fischer, T. S. Wang, G. Yenokyan, S. Kang and A. L. Chien, Association of indoor tanning frequency with risky sun protection practices and skin cancer screening, JAMA Dermatol., 2017, 153, 168–174 CrossRef PubMed.
- M. C. Kirchberger, M. V. Heppt, T. K. Eigentler, M. A. Kirchberger, G. Schuler and L. Heinzerling, The tanning habits and interest in sunscreen of Google users: what happened in 12 years?, Photodermatol., Photoimmunol. Photomed., 2017, 33, 68–74 CrossRef PubMed.
- S. T. Shih, R. Carter, S. Heward and C. Sinclair, Economic evaluation of future skin cancer prevention in Australia, Prev. Med., 2017, 99, 7–12 CrossRef PubMed.
- M. Fransen, A. Karahalios, N. Sharma, D. R. English, G. G. Giles and R. D. Sinclair, Non-melanoma skin cancer in Australia, Med. J. Aust., 2012, 197, 565–568 CrossRef PubMed.
- S. T. F. Shih, R. Carter, S. Heward and C. Sinclair, Skin cancer has a large impact on our public hospitals but prevention programs continue to demonstrate strong economic credentials, Aust. N. Z. J. Public Health, 2017, 41, 371–376 CrossRef PubMed.
- L. Pil, I. Hoorens, K. Vossaert, V. Kruse, I. Tromme, N. Speybroeck, L. Brochez and L. Annemans, Burden of skin cancer in Belgium and cost-effectiveness of primary prevention by reducing ultraviolet exposure, Prev. Med., 2016, 93, 177–182 CrossRef PubMed.
- P. H. Hart, S. Gorman and J. J. Finlay-Jones, Modulation of the immune system by UV radiation: more than just the effects of vitamin D?, Nat. Rev. Immunol., 2011, 11, 584–596 CrossRef CAS PubMed.
- V. Patra, S. N. Byrne and P. Wolf, The skin microbiome: is it affected by UV-induced immune suppression?, Front. Microbiol., 2016, 7, 1235 Search PubMed.
- R. Prasad and S. K. Katiyar, Crosstalk among UV-induced inflammatory mediators, DNA damage and epigenetic regulators facilitates suppression of the immune system, Photochem. Photobiol., 2017, 93, 930–936 CrossRef CAS PubMed.
- M. Bustamante, C. Hernandez-Ferrer, Y. Sarria, G. I. Harrison, L. Nonell, W. Kang, M. R. Friedlander, X. Estivill, J. R. Gonzalez, M. Nieuwenhuijsen and A. R. Young, The acute effects of ultraviolet radiation on the blood transcriptome are independent of plasma 25OHD3, Environ. Res., 2017, 159, 239–248 CrossRef CAS PubMed.
- M. P. Ponda, Y. Liang, J. Kim, R. Hutt, K. Dowd, P. Gilleaudeau, M. M. Sullivan-Whalen, T. Rodrick, D. J. Kim, I. Barash, M. A. Lowes and J. L. Breslow, A randomized clinical trial in vitamin D-deficient adults comparing replenishment with oral vitamin D3 with narrow-band UV type B light: effects on cholesterol and the transcriptional profiles of skin and blood, Am. J. Clin. Nutr., 2017, 105, 1230–1238 CrossRef CAS PubMed.
- K. Mahendraraj, S. Shrestha, C. S. Lau and R. S. Chamberlain, Ocular melanoma-when you have seen one, you have not seen them all: a clinical outcome study from the Surveillance, Epidemiology and End Results (SEER) database (1973–2012), Clin. Ophthalmol., 2017, 11, 153–160 CrossRef PubMed.
- A. Amaro, R. Gangemi, F. Piaggio, G. Angelini, G. Barisione, S. Ferrini and U. Pfeffer, The biology of uveal melanoma., Cancer. Metastasis. Rev., 2017, 36, 109–140 CrossRef PubMed.
- C. Rivolta, B. Royer-Bertrand, D. Rimoldi, A. Schalenbourg, L. Zografos, S. Leyvraz and A. Moulin, UV light signature in conjunctival melanoma; not only skin should be protected from solar radiation., J. Hum. Genet., 2016, 61, 361–362 CrossRef CAS PubMed.
- T. Nayman, C. Bostan, P. Logan and M. N. Burnier, Uveal melanoma risk factors: a systematic review of meta-analyses., Curr. Eye. Res., 2017, 42, 1085–1093 CrossRef PubMed.
- C. Schwab, C. Mayer, I. Zalaudek, R. Riedl, M. Richtig, W. Wackernagel, R. Hofmann-Wellenhof, G. Richtig, G. Langmann, L. Tarmann, A. Wedrich and E. Richtig, Iris freckles a potential biomarker for chronic sun damage., Invest. Ophthalmol. Visual Sci., 2017, 58, BIO174–BIO179 Search PubMed.
- K. M. Haworth and H. L. Chandler, Seasonal effect on ocular sun exposure and conjunctival uv autofluorescence, Optom. Vis. Sci., 2017, 94, 219–228 CrossRef PubMed.
- S. Kearney, L. O'Donoghue, L. K. Pourshahidi, P. M. Richardson and K. J. Saunders, The use of conjunctival ultraviolet autofluorescence (CUVAF) as a biomarker of time spent outdoors., Ophthalmic Physiol. Opt., 2016, 36, 359–369 CrossRef PubMed.
- C. Sun, A. Pezic, D. A. Mackey, J. B. Carlin, A. Kemp, J. A. Ellis, F. J. Cameron, C. P. Rodda, T. Dwyer, M. T. Coroneo and A.-L. Ponsonby, Conjunctival ultraviolet autofluorescence as a measure of past sun exposure in children., Cancer Epidemiol., Biomarkers Prev., 2017, 26, 1146–1153 CrossRef CAS PubMed.
- D. Pascolini and S. P. Mariotti, Global estimates of visual impairment: 2010, Br. J. Opthalmol., 2012, 96, 614–618 CrossRef PubMed.
- N. Congdon, B. O'Colmain, C. C. Klaver, R. Klein, B. Munoz, D. S. Friedman, J. Kempen, H. R. Taylor, P. Mitchell and G. Eye Diseases Prevalence Research, Causes and prevalence of visual impairment among adults in the United States, Arch. Ophthalmol., 2004, 122, 477–485 CrossRef PubMed.
- T. Schick, L. Ersoy, Y. T. E. Lechanteur, N. T. M. Saksens, C. B. Hoyng, A. I. den Hollander, B. Kirchhof and S. Fauser, History of sunlight exposure is a risk factor for age-related macular degeneration, Retina, 2016, 36, 787–790 CrossRef CAS PubMed.
- G. J. McKay, I. S. Young, A. McGinty, G. C. G. Bentham, U. Chakravarthy, M. Rahu, J. Seland, G. Soubrane, L. Tomazzoli, F. Topouzis, J. Vioque, P. T. de Jong and A. E. Fletcher, Associations between serum vitamin D and genetic variants in vitamin d pathways and age-related macular degeneration in the European Eye Study, Ophthalmol., 2017, 124, 90–96 CrossRef PubMed.
- S. Mesrine, M. Kvaskoff, T. Bah, L. Wald, F. Clavel-Chapelon and M. C. Boutron-Ruault, Nevi, ambient ultraviolet radiation, and thyroid cancer risk: a French prospective study, Epidemiology, 2017, 28, 694–702 CrossRef PubMed.
- X. Chen, H. Chen, W. Cai, M. Maguire, B. Ya, F. Zuo, R. Logan, H. Li, K. Robinson, C. R. Vanderburg, Y. Yu, Y. Wang, D. E. Fisher and M. A. Schwarzschild, The melanoma-linked “redhead” MC1R influences dopaminergic neuron survival, Ann. Neurol., 2017, 81, 395–406 CrossRef CAS PubMed.
- L. A. Dalvin, G. M. Damento, B. P. Yawn, B. A. Abbott, D. O. Hodge and J. S. Pulido, Parkinson disease and melanoma: Confirming and reexamining an association, Mayo Clin. Proc., 2017, 92, 1070–1079 CrossRef PubMed.
- C. R. Medici, C. H. Vestergaard, D. Hadzi-Pavlovic, P. Munk-Jorgensen and G. Parker, Seasonal variations in hospital admissions for mania: Examining for associations with weather variables over time, J. Affective Disord., 2016, 205, 81–86 CrossRef PubMed.
- A. R. Young, J. Claveau and A. B. Rossi, Ultraviolet radiation and the skin: Photobiology and sunscreen photoprotection, J. Am. Acad. Dermatol., 2017, 76, S100–S109 CrossRef CAS PubMed.
- C. M. Olsen, L. F. Wilson, A. C. Green, N. Biswas, J. Loyalka and D. C. Whiteman, Prevention of DNA damage in human skin by topical sunscreens, Photodermatol., Photoimmunol. Photomed., 2017, 33, 135–142 CrossRef CAS PubMed.
- M. Randhawa, S. Wang, J. J. Leyden, G. O. Cula, A. Pagnoni and M. D. Southall, Daily use of a facial broad spectrum sunscreen over one-year significantly improves clinical evaluation of photoaging, Dermatol. Surg., 2016, 42, 1354–1361 CrossRef CAS PubMed.
- S. M. Herzog, H. W. Lim, M. S. Williams, I. D. de Maddalena, U. Osterwalder and C. Surber, Sun protection factor communication of sunscreen effectiveness: A web-based study of perception of effectiveness by dermatologists, JAMA Dermatol., 2017, 153, 348–350 CrossRef PubMed.
- U. Osterwalder, M. Sohn and B. Herzog, Global state of sunscreens, Photodermatol., Photoimmunol. Photomed., 2014, 30, 62–80 CrossRef.
- B. L. Diffey, Optimizing the spectral absorption profile of sunscreens, Int. J. Cosmet. Sci., 2017, 39, 90–92 CrossRef CAS PubMed.
- B. Diffey and U. Osterwalder, Labelled sunscreen SPFs may overestimate protection in natural sunlight, Photochem. Photobiol. Sci., 2017, 16, 1519–1523 CAS.
- I. M. Heerfordt, P. A. Philipsen, B. O. Larsen and H. C. Wulf, Long-term trend in sunscreen use among beachgoers in Denmark, Acta Derm.-Venereol., 2017, 97, 1202–1205 CrossRef PubMed.
- Z. Jovanovic, T. Schornstein, A. Sutor, G. Neufang and R. Hagens, Conventional sunscreen application does not lead to sufficient body coverage, Int. J. Cosmet. Sci., 2017, 39, 550–555 CrossRef CAS PubMed.
- H. Ou-Yang, L. I. Jiang, K. Meyer, S. Q. Wang, A. S. Farberg and D. S. Rigel, Sun protection by beach umbrella vs sunscreen with a high sun protection factor: A randomized clinical trial, JAMA Dermatol., 2017, 153, 304–308 CrossRef PubMed.
- R. B. Weller, Sunlight has cardiovascular benefits independently of vitamin D, Blood Purif., 2016, 41, 130–134 CrossRef CAS PubMed.
- G. Holliman, D. Lowe, H. Cohen, S. Felton and K. Raj, Ultraviolet radiation-induced production of nitric oxide: a multi-cell and multi-donor analysis, Sci. Rep., 2017, 7, 11105 CrossRef PubMed.
- N. Sasaki, T. Yamashita, K. Kasahara, A. Fukunaga, T. Yamaguchi, T. Emoto, K. Yodoi, T. Matsumoto, K. Nakajima, T. Kita, M. Takeda, T. Mizoguchi, T. Hayashi, Y. Sasaki, M. Hatakeyama, K. Taguchi, K. Washio, S. Sakaguchi, B. Malissen, C. Nishigori and K. I. Hirata, UVB exposure prevents atherosclerosis by regulating immunoinflammatory responses, Arterioscler., Thromb., Vasc. Biol., 2017, 37, 66–74 CrossRef CAS PubMed.
- P. G. Lindqvist and M. Landin-Olsson, The relationship between sun exposure and all-cause mortality, Photochem. Photobiol. Sci., 2017, 16, 354–361 CAS.
- C. Skobowiat, A. E. Postlethwaite and A. T. Slominski, Skin exposure to ultraviolet B rapidly activates systemic neuroendocrine and immunosuppressive responses, Photochem. Photobiol., 2017, 93, 1008–1015 CrossRef CAS PubMed.
- C. Ekmekcioglu, D. Haluza and M. Kundi, 25-hydroxyvitamin D status and risk for colorectal cancer and type 2 diabetes mellitus: A systematic review and meta-analysis of epidemiological studies, Int. J. Environ. Res. Public Health, 2017, 14, 127 CrossRef PubMed.
- R. Zhang, B. Li, X. Gao, R. Tian, Y. Pan, Y. Jiang, H. Gu, Y. Wang, Y. Wang and G. Liu, Serum 25-hydroxyvitamin D and the risk of cardiovascular disease: dose-response meta-analysis of prospective studies, Am. J. Clin. Nutr., 2017, 105, 810–819 CrossRef CAS PubMed.
- A. M. Goodwill and C. Szoeke, A systematic review and meta-analysis of the effect of low vitamin D on cognition, J. Am. Geriatr. Soc., 2017, 65, 2161–2168S CrossRef PubMed.
- I. Sommer, U. Griebler, C. Kien, S. Auer, I. Klerings, R. Hammer, P. Holzer and G. Gartlehner, Vitamin D deficiency as a risk factor for dementia: A systematic review and meta-analysis, BMC Geriatr., 2017, 17, 16 CrossRef PubMed.
- L. L. Qin, F. G. Lu, S. H. Yang, H. L. Xu and B. A. Luo, Does maternal vitamin D deficiency increase the risk of preterm birth: a meta-analysis of observational studies, Nutrients, 2016, 8, 301 CrossRef PubMed.
- H. Feng, P. Xun, K. Pike, A. K. Wills, B. L. Chawes, H. Bisgaard, W. Cai, Y. Wan and K. He, In utero exposure to 25-hydroxyvitamin D and risk of childhood asthma, wheeze, and respiratory tract infections: A meta-analysis of birth cohort studies, J. Allergy Clin. Immunol., 2017, 139, 1508–1517 CrossRef CAS PubMed.
- P. Autier, P. Mullie, A. Macacu, M. Dragomir, M. Boniol, K. Coppens, C. Pizot and M. Boniol, Effect of vitamin D supplementation on non-skeletal disorders: a systematic review of meta-analyses and randomised trials, Lancet Diab. Endocrinol., 2017, 5(12), 986–1004 CrossRef.
- A. R. Martineau, D. A. Jolliffe, R. L. Hooper, L. Greenberg, J. F. Aloia, P. Bergman, G. Dubnov-Raz, S. Esposito, D. Ganmaa, A. A. Ginde, E. C. Goodall, C. C. Grant, C. J. Griffiths, W. Janssens, I. Laaksi, S. Manaseki-Holland, D. Mauger, D. R. Murdoch, R. Neale, J. R. Rees, S. Simpson Jr., I. Stelmach, G. T. Kumar, M. Urashima and C. A. Camargo Jr., Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data, Br. Med. J., 2017, 356, i6583 CrossRef PubMed.
- J. S. Ong, G. Cuellar-Partida, Y. Lu, S. Australian Ovarian Cancer, P. A. Fasching, A. Hein, S. Burghaus, M. W. Beckmann, D. Lambrechts, E. Van Nieuwenhuysen, I. Vergote, A. Vanderstichele, J. Anne Doherty, M. Anne Rossing, J. Chang-Claude, U. Eilber, A. Rudolph, S. Wang-Gohrke, M. T. Goodman, N. Bogdanova, T. Dork, M. Durst, P. Hillemanns, I. B. Runnebaum, N. Antonenkova, R. Butzow, A. Leminen, H. Nevanlinna, L. M. Pelttari, R. P. Edwards, J. L. Kelley, F. Modugno, K. B. Moysich, R. B. Ness, R. Cannioto, E. Hogdall, C. K. Hogdall, A. Jensen, G. G. Giles, F. Bruinsma, S. K. Kjaer, M. A. Hildebrandt, D. Liang, K. H. Lu, X. Wu, M. Bisogna, F. Dao, D. A. Levine, D. W. Cramer, K. L. Terry, S. S. Tworoger, M. Stampfer, S. Missmer, L. Bjorge, H. B. Salvesen, R. K. Kopperud, K. Bischof, K. K. Aben, L. A. Kiemeney, L. F. Massuger, A. Brooks-Wilson, S. H. Olson, V. McGuire, J. H. Rothstein, W. Sieh, A. S. Whittemore, L. S. Cook, N. D. Le, C. B. Gilks, J. Gronwald, A. Jakubowska, J. Lubinski, T. Kluz, H. Song, J. P. Tyrer, N. Wentzensen, L. Brinton, B. Trabert, J. Lissowska, J. R. McLaughlin, S. A. Narod, C. Phelan, H. Anton-Culver, A. Ziogas, D. Eccles, I. Campbell, S. A. Gayther, A. Gentry-Maharaj, U. Menon, S. J. Ramus, A. H. Wu, A. Dansonka-Mieszkowska, J. Kupryjanczyk, A. Timorek, L. Szafron, J. M. Cunningham, B. L. Fridley, S. J. Winham, E. V. Bandera and E. M. Poole, et al., Association of vitamin D levels and risk of ovarian cancer: a Mendelian randomization study, Int. J. Epidemiol., 2016, 45, 1619–1630 CrossRef PubMed.
- B. Rhead, M. Baarnhielm, M. Gianfrancesco, A. Mok, X. Shao, H. Quach, L. Shen, C. Schaefer, J. Link, A. Gyllenberg, A. K. Hedstrom, T. Olsson, J. Hillert, I. Kockum, M. M. Glymour, L. Alfredsson and L. F. Barcellos, Mendelian randomization shows a causal effect of low vitamin D on multiple sclerosis risk, Neurol.: Genet., 2016, 2, e97 CrossRef PubMed.
- M. A. Gianfrancesco, P. Stridh, B. Rhead, X. Shao, E. Xu, J. S. Graves, T. Chitnis, A. Waldman, T. Lotze, T. Schreiner, A. Belman, B. Greenberg, B. Weinstock-Guttman, G. Aaen, J. M. Tillema, J. Hart, S. Caillier, J. Ness, Y. Harris, J. Rubin, M. Candee, L. Krupp, M. Gorman, L. Benson, M. Rodriguez, S. Mar, I. Kahn, J. Rose, S. Roalstad, T. C. Casper, L. Shen, H. Quach, D. Quach, J. Hillert, M. Baarnhielm, A. Hedstrom, T. Olsson, I. Kockum, L. Alfredsson, C. Metayer, C. Schaefer, L. F. Barcellos, E. Waubant and C. Network of Pediatric Multiple Sclerosis, Evidence for a causal relationship between low vitamin D, high BMI, and pediatric-onset MS, Neurology, 2017, 88, 1623–1629 CrossRef CAS PubMed.
- L. E. Mokry, S. Ross, J. A. Morris, D. Manousaki, V. Forgetta and J. B. Richards, Genetically decreased vitamin D and risk of Alzheimer disease, Neurology, 2016, 87, 2567–2574 CrossRef CAS PubMed.
- E. B. Hysinger, J. D. Roizen, F. D. Mentch, L. Vazquez, J. J. Connolly, J. P. Bradfield, B. Almoguera, P. M. Sleiman, J. L. Allen, M. A. Levine and H. Hakonarson, Mendelian randomization analysis demonstrates that low vitamin D is unlikely causative for pediatric asthma, J. Allergy Clin. Immunol., 2016, 138, 1747–1749 CrossRef PubMed.
- D. Manousaki, L. Paternoster, M. Standl, M. F. Moffatt, M. Farrall, E. Bouzigon, D. P. Strachan, F. Demenais, M. Lathrop, W. Cookson and J. B. Richards, Vitamin D levels and susceptibility to asthma, elevated immunoglobulin E levels, and atopic dermatitis: A Mendelian randomization study, PLoS Med., 2017, 14, e1002294 Search PubMed.
- D. Manousaki, L. E. Mokry, S. Ross, D. Goltzman and J. B. Richards, Mendelian randomization studies do not support a role for vitamin D in coronary artery disease, Circ.: Cardiovasc. Genet., 2016, 9, 349–356 CAS.
- P. Brondum-Jacobsen, M. Benn, S. Afzal and B. G. Nordestgaard, No evidence that genetically reduced 25-hydroxyvitamin D is associated with increased risk of ischaemic heart disease or myocardial infarction: a Mendelian randomization study, Int. J. Epidemiol., 2015, 44, 651–661 CrossRef PubMed.
- S. C. Larsson, A. B. Singleton, M. A. Nalls, J. B. Richards and C. International Parkinson's Disease Genomics, No clear support for a role for vitamin D in Parkinson's disease: A Mendelian randomization study, Mov. Disord., 2017, 32, 1249–1252 CrossRef CAS PubMed.
- A. E. Taylor, S. Burgess, J. J. Ware, S. H. Gage, J. B. Richards, G. Davey Smith and M. R. Munafo, Investigating causality in the association between 25(OH)D and schizophrenia, Sci. Rep., 2016, 6, 26496 CrossRef CAS PubMed.
- P. Datta, P. A. Philipsen, P. Olsen, B. Petersen, P. Johansen, N. Morling and H. C. Wulf, Major inter-personal variation in the increase and maximal level of 25-hydroxy vitamin D induced by UVB, Photochem. Photobiol. Sci., 2016, 15, 536–545 CAS.
- L. E. Rhodes, A. R. Webb, H. I. Fraser, R. Kift, M. T. Durkin, D. Allan, S. J. O'Brien, A. Vail and J. L. Berry, Recommended summer sunlight exposure levels can produce sufficient (≥ 20 ng ml−1) but not the proposed optimal (≥ 32 ng ml−1) 25(OH)D levels at UK latitudes, J. Invest. Dermatol., 2010, 130, 1411–1418 CrossRef CAS PubMed.
- P. Datta, P. A. Philipsen, P. Olsen, M. K. Bogh, P. Johansen, A. V. Schmedes, N. Morling and H. C. Wulf, The half-life of 25(OH)D after UVB exposure depends on gender and vitamin D receptor polymorphism but mainly on the start level, Photochem. Photobiol. Sci., 2017, 16, 985–995 CAS.
- C. M. O'Neill, A. Kazantzidis, M. J. Ryan, N. Barber, C. T. Sempos, R. A. Durazo-Arvizu, R. Jorde, G. Grimnes, G. Eiriksdottir, V. Gudnason, M. F. Cotch, M. Kiely, A. R. Webb and K. D. Cashman, Seasonal changes in vitamin D-effective UVB availability in Europe and associations with population serum 25-Hydroxyvitamin D, Nutrients, 2016, 8, 533 CrossRef PubMed.
- T. Jaaskelainen, S. T. Itkonen, A. Lundqvist, M. Erkkola, T. Koskela, K. Lakkala, K. G. Dowling, G. L. Hull, H. Kroger, J. Karppinen, E. Kyllonen, T. Harkanen, K. D. Cashman, S. Mannisto and C. Lamberg-Allardt, The positive impact of general vitamin D food fortification policy on vitamin D status in a representative adult Finnish population: evidence from an 11-y follow-up based on standardized 25-hydroxyvitamin D data, Am. J. Clin. Nutr., 2017, 105, 1512–1520 Search PubMed.
- K. M. Williams, G. C. G. Bentham, I. S. Young, A. McGinty, G. J. McKay, R. Hogg, C. J. Hammond, U. Chakravarthy, M. Rahu, J. Seland, G. Soubrane, L. Tomazzoli, F. Topouzis and A. E. Fletcher, Association between myopia, ultraviolet B radiation exposure, serum vitamin D concentrations, and genetic polymorphisms in vitamin D metabolic pathways in a multicountry European study, JAMA Ophthalmol., 2017, 135, 47–53 CrossRef PubMed.
- H. Torii, T. Kurihara, Y. Seko, K. Negishi, K. Ohnuma, T. Inaba, M. Kawashima, X. Jiang, S. Kondo, M. Miyauchi, Y. Miwa, Y. Katada, K. Mori, K. Kato, K. Tsubota, H. Goto, M. Oda, M. Hatori and K. Tsubota, Violet light exposure can be a preventive strategy against myopia progression, EBioMedicine, 2017, 15, 210–219 CrossRef PubMed.
- F. Schaeffel and E. L. Smith, Inhibiting myopia by (nearly) invisible light?, EBioMedicine, 2017, 16, 27–28 CrossRef PubMed.
- D. Fajuyigbe and A. R. Young, The impact of skin colour on human photobiological responses, Pigment Cell Melanoma Res., 2016, 29, 607–618 CrossRef CAS PubMed.
- R. K. Marwaha, V. Sreenivas, D. Talwar, V. K. Yenamandra, A. Challa, R. Lakshmy, V. K. Sharma and G. Sethuraman, Impact of solar ultraviolet B radiation (290–320 nm) on vitamin D synthesis in children with type IV and V skin, Br. J. Dermatol., 2015, 173, 604–606 CrossRef CAS PubMed.
- O. A. Hakim, K. Hart, P. McCabe, J. Berry, R. Francesca, L. E. Rhodes, N. Spyrou, A. Alfuraih and S. Lanham-New, Vitamin D production in UK Caucasian and South Asian women following UVR exposure, J. Steriod Biochem. Mol. Biol., 2016, 164, 223–229 CrossRef CAS PubMed.
- B. Petersen, H. C. Wulf, M. Triguero-Mas, P. A. Philipsen, E. Thieden, P. Olsen, J. Heydenreich, P. Dadvand, X. Basagana, T. S. Liljendahl, G. I. Harrison, D. Segerback, A. W. Schmalwieser, A. R. Young and M. J. Nieuwenhuijsen, Sun and ski holidays improve vitamin D status, but are associated with high levels of DNA damage, J. Invest. Dermatol., 2014, 134, 2806–2813 CrossRef CAS PubMed.
- S. J. Felton, M. S. Cooke, R. Kift, J. L. Berry, A. R. Webb, P. M. Lam, F. R. de Gruijl, A. Vail and L. E. Rhodes, Concurrent beneficial (vitamin D production) and hazardous (cutaneous DNA damage) impact of repeated low-level summer sunlight exposures, Br. J. Dermatol., 2016, 175, 1320–1328 CrossRef CAS PubMed.
- C. Ulrich, C. Salavastru, T. Agner, A. Bauer, R. Brans, M. N. Crepy, K. Ettler, F. Gobba, M. Goncalo, B. Imko-Walczuk, J. Lear, J. Macan, A. Modenese, J. Paoli, P. Sartorelli, K. Stageland, P. Weinert, N. Wroblewski, H. C. Wulf and S. M. John, The European Status Quo in legal recognition and patient-care services of occupational skin cancer, J. Eur. Acad. Dermatol. Venereol., 2016, 30(Suppl 3), 46–51 CrossRef PubMed.
- R. Harari Arjona, J. Pineiros, M. Ayabaca and F. Harari Freire, Climate change and agricultural workers’ health in Ecuador: occupational exposure to UV radiation and hot environments, Ann. Ist. Super. Sanita., 2016, 52, 368–373 Search PubMed.
- United States Department of Labor Bureau of Labor Statistics, Employment Projections: Fasting Growing Occupations, https://www.bls.gov/emp/ep_table_103.htm, updated October 24, 2017, accessed December 7, 2017 Search PubMed.
- M. Norval, R. M. Lucas, A. P. Cullen, F. R. de Gruijl, J. Longstreth, Y. Takizawa and J. C. van der Leun, The human health effects of ozone depletion and interactions with climate change, Photochem. Photobiol. Sci., 2011, 10, 199–225 CAS.
- D. Michal Freedman, C. M. Kitahara, M. S. Linet, B. H. Alexander, G. Neta, M. P. Little and E. K. Cahoon, Ambient temperature and risk of first primary basal cell carcinoma: A nationwide United States cohort study, J. Photochem. Photobiol., B., 2015, 148, 284–289 CrossRef CAS PubMed.
- C. E. Lan, Y. T. Wang, C. Y. Lu, A. H. Fang and C. S. Wu, The effect of interaction of heat and UVB on human keratinocyte: Novel insights on UVB-induced carcinogenesis of the skin, J. Dermatol. Sci., 2017, 88, 207–215 CrossRef CAS PubMed.
- E. P. Lim, H. H. Hendon, J. M. Arblaster, F. Delage, H. Nguyen, S. K. Min and M. C. Wheeler, The impact of the Southern Annular Mode on future changes in Southern Hemisphere rainfall, Geophys. Res. Lett., 2016, 43, 7160–7167 CrossRef.
- E. P. Lim, H. H. Hendon, J. M. Arblaster, C. Chung, A. F. Moise, P. Hope, G. Young and M. Zhao, Interaction of the recent 50 years SST trend and La Niña 2010: amplification of the Southern Annular Mode and Australian springtime rainfall, Clim. Dyn., 2016, 47, 2273–2291 CrossRef.
- H. N. Duc, K. Rivett, K. MacSween and L. A. Linh, Association of climate drivers with rainfall in New South Wales, Australia, using Bayesian Model Averaging, Theoret. Appl. Climatol., 2017, 127, 169–185 CrossRef.
- S. Bronnimann, M. Jacques-Coper, E. Rozanov, A. M. Fischer, O. Morgenstern, G. Zeng, H. Akiyoshi and Y. Yamashita, Tropical circulation and precipitation response to ozone depletion and recovery, Environ. Res. Lett., 2017, 12, 064011 CrossRef.
- K. R. Clem, J. A. Renwick and J. McGregor, Relationship between eastern tropical Pacific cooling and recent trends in the Southern Hemisphere zonal-mean circulation, Clim. Dyn., 2017, 49, 113–129 CrossRef.
- D. A. Chaves, G. B. Lyra, M. R. Francelino, L. D. B. Silva, A. Thomazini and C. Schaefer, Active layer and permafrost thermal regime in a patterned ground soil in Maritime Antarctica, and relationship with climate variability models, Sci. Total Environ., 2017, 584, 572–585 CrossRef PubMed.
- D. Manatsa, C. Mudavanhu, T. D. Mushore and E. Mavhura, Linking major shifts in East Africa ‘short rains’ to the Southern Annular Mode, Int. J. Climatol., 2016, 36, 1590–1599 CrossRef.
- N. J. Abram, R. Mulvaney, F. Vimeux, S. J. Phipps, J. Turner and M. H. England, Evolution of the Southern Annular Mode during the past millennium, Nat. Clim. Change, 2014, 4, 564–569 CrossRef CAS.
- S. A. Robinson and D. J. Erickson III, Not just about sunburn–the ozone hole's profound effect on climate has significant implications for Southern Hemisphere ecosystems, Glob. Change Biol., 2015, 21, 515–527 CrossRef PubMed.
- J. F. Bornman, P. W. Barnes, S. A. Robinson, C. L. Ballaré, S. D. Flint and M. M. Caldwell, Solar ultraviolet radiation and ozone depletion-driven climate change: effects on terrestrial ecosystems, Photochem. Photobiol. Sci., 2015, 14, 88–107 CAS.
- C. Le Quéré, C. Rödenbeck, E. T. Buitenhuis, T. J. Conway, R. Langenfelds, A. Gomez, C. Labuschagne, M. Ramonet, T. Nakazawa, N. Metzl, N. Gillett and M. Heimann, Saturation of the southern ocean CO2 sink due to recent climate change, Science, 2007, 316, 1735–1738 CrossRef PubMed.
- J. Perlwitz, Tug of war on the jet stream, Nat. Clim. Change, 2011, 1, 29–31 CrossRef.
- NASA, Ozone Hole Watch, National Aeronautics and Space Administration. Goddard Space Flight Center, http://ozonewatch.gsfc.nasa.gov/, updated October 30, 2017, accessed October 30, 2017 Search PubMed.
- A. Holz, J. Paritsis, I. A. Mundo, T. T. Veblen, T. Kitzberger, G. J. Williamson, E. Aráoz, C. Bustos-Schindler, M. E. González, H. R. Grau and J. M. Quezada, Southern Annular Mode drives multicentury wildfire activity in southern South America, Proc. Natl. Acad. Sci. U. S. A., 2017, 114, 9552–9557 CrossRef CAS PubMed.
- A. A. Munoz, A. Gonzalez-Reyes, A. Lara, D. Sauchyn, D. Christie, P. Puchi, R. Urrutia-Jalabert, I. Toledo-Guerrero, I. Aguilera-Betti, I. Mundo, P. R. Sheppard, D. Stahle, R. Villalba, P. Szejner, C. LeQuesne and J. Vanstone, Streamflow variability in the Chilean Temperate-Mediterranean climate transition (35 degrees S-42 degrees S) during the last 400 years inferred from tree-ring records, Clim. Dyn., 2016, 47, 4051–4066 CrossRef.
- C. Coviaga, A. Rizzo, P. Perez, R. Daga, D. Poire, G. Cusminsky and S. R. Guevara, Reconstruction of the hydrologic history of a shallow Patagonian steppe lake during the past 700 yr, using chemical, geologic, and biological proxies, Quat. Res., 2017, 87, 208–226 CrossRef CAS.
- T. S. M. Randriamahefasoa and C. J. C. Reason, Interannual variability of rainfall characteristics over southwestern Madagascar, Theoret. Appl. Climatol., 2017, 128, 421–437 CrossRef.
- L. Lopez, D. Stahle, R. Villalba, M. Torbenson, S. Feng and E. Cook, Tree ring reconstructed rainfall over the southern Amazon Basin, Geophys. Res. Lett., 2017, 44, 7410–7418 CrossRef.
- K. R. Clem, J. A. Renwick, J. McGregor and R. L. Fogt, The relative influence of ENSO and SAM on Antarctic Peninsula climate, J. Geophys. Res. Atmos., 2016, 121, 9324–9341 Search PubMed.
- M. J. Amesbury, T. P. Roland, J. Royles, D. A. Hodgson, P. Convey, H. Griffiths and D. J. Charman, Widespread biological response to rapid warming on the Antarctic peninsula, Curr. Biol., 2017, 27, 1616–1622.e2 CrossRef CAS PubMed.
- J. Turner, H. Lu, I. White, J. C. King, T. Phillips, J. S. Hosking, T. J. Bracegirdle, G. J. Marshall, R. Mulvaney and P. Deb, Absence of 21st century warming on Antarctic Peninsula consistentwith natural variability, Nature, 2016, 535, 411–415 CrossRef CAS PubMed.
- R. Fay, C. Barbraud, K. Delord and H. Weimerskirch, Contrasting effects of climate and population density over time and life stages in a long-lived seabird, Funct. Ecol., 2017, 31, 1275–1284 CrossRef PubMed.
- H. Weimerskirch, M. Louzao, S. de Grissac and K. Delord, Changes in wind pattern alter Albatross distribution and life-history traits, Science, 2012, 335, 211–214 CrossRef CAS PubMed.
- C. R. McMahon, R. G. Harcourt, H. R. Burton, O. Daniel and M. A. Hindell, Seal mothers expend more on offspring under favourable conditions and less when resources are limited, J. Anim. Ecol., 2017, 86, 359–370 CrossRef PubMed.
- F. Abadi, C. Barbraud and O. Gimenez, Integrated population modeling reveals the impact of climate on the survival of juvenile emperor penguins, Glob. Change Biol., 2017, 23, 1353–1359 CrossRef PubMed.
- G. T. Pecl, M. B. Araújo, J. D. Bell, J. Blanchard, T. C. Bonebrake, I.-C. Chen, T. D. Clark, R. K. Colwell, F. Danielsen, B. Evengård, L. Falconi, S. Ferrier, S. Frusher, R. A. Garcia, R. B. Griffis, A. J. Hobday, C. Janion-Scheepers, M. A. Jarzyna, S. Jennings, J. Lenoir, H. I. Linnetved, V. Y. Martin, P. C. McCormack, J. McDonald, N. J. Mitchell, T. Mustonen, J. M. Pandolfi, N. Pettorelli, E. Popova, S. A. Robinson, B. R. Scheffers, J. D. Shaw, C. J. B. Sorte, J. M. Strugnell, J. M. Sunday, M.-N. Tuanmu, A. Vergés, C. Villanueva, T. Wernberg, E. Wapstra and S. E. Williams, Biodiversity redistribution under climate change: Impacts on ecosystems and human well-being, Science, 2017, 355, eaai9214 CrossRef PubMed.
- T. M. Robson, S. M. Hartikainen and P. J. Aphalo, How does solar ultraviolet-B radiation improve drought tolerance of silver birch (Betula pendula Roth.) seedlings?, Plant Cell Environ., 2015, 38, 953–967 CrossRef CAS PubMed.
- R. Alonso, F. J. Berli, P. Piccoli and R. Bottini, Ultraviolet-B radiation, water deficit and abscisic acid: a review of independent and interactive effects on grapevines, Theoret. Exp. Plant Physiol., 2016, 28, 11–22 CrossRef CAS.
- B. Mao, Y. Wang, T.-H. Zhao, R.-R. Tian, W. Wang and J.-S. Ye, Combined effects of elevated O3 concentrations and enhanced UV-B radiation of the biometric and biochemical properties of soybean roots, Front. Plant Sci., 2017, 8, 1568 CrossRef PubMed.
- C. van Leeuwen and P. Darriet, The impact of climate change on viticulture and wine quality, J. Wine Econom., 2016, 11, 150–167 CrossRef.
- M.-Á. Del-Castillo-Alonso, M. P. Diago, R. Tomás-Las-Heras, L. Monforte, G. Soriano, J. Martínez-Abaigar and E. Núñez-Olivera, Effects of ambient solar UV radiation on grapevine leaf physiology and berry phenolic composition along one entire season under Mediterranean field conditions, Plant Physiol. Biochem., 2016, 109, 374–386 CrossRef CAS PubMed.
- A. Luengo Escobar, M. Alberdi, P. Acevedo, M. Machado, A. Nunes-Nesi, C. Inostroza-Blancheteau and M. Reyes-Díaz, Distinct physiological and metabolic reprogramming by highbush blueberry (Vaccinium corymbosum) cultivars revealed during long-term UV-B radiation, Physiol. Plant., 2017, 160, 46–64 CrossRef CAS PubMed.
- S. Stark, M. Vaisanen, H. Ylanne, R. Julkunen-Tiitto and F. Martz, Decreased phenolic defence in dwarf birch (Betula nana) after warming in subarctic tundra, Polar Biol., 2015, 38, 1993–2005 CrossRef.
- C. Joubert, P. R. Young, H. A. Eyéghé-Bickong and M. A. Vivier, Field-grown grapevine berries use carotenoids and the associated xanthophyll cycles to acclimate to UV exposure differentially in high and low light (shade) conditions, Front. Plant Sci., 2016, 7, 786 Search PubMed.
- L. Zoratti, K. Karppinen, A. L. Escobar, H. Haggman and L. Jaakola, Light-controlled flavonoid biosynthesis in fruits, Front. Plant Sci., 2014, 5, 534 Search PubMed.
- P. Carbonell-Bejerano, M. P. Diago, J. Martinez-Abaigar, J. M. Martinez-Zapater, J. Tardaguila and E. Nunez-Olivera, Solar ultraviolet radiation is necessary to enhance grapevine fruit ripening transcriptional and phenolic responses, BMC Plant Biol., 2014, 14, 183 CrossRef PubMed.
- M. Ordidge, P. Garcia-Macias, N. H. Battey, M. H. Gordon, P. Hadley, P. John, J. A. Lovegrove, E. Vysini and A. Wagstaffe, Phenolic contents of lettuce, strawberry, raspberry, and blueberry crops cultivated under plastic films varying in ultraviolet transparency, Food Chem., 2010, 119, 1224–1227 CrossRef CAS.
- C. Pastore, S. Zenoni, M. Fasoli, M. Pezzotti, G. B. Tornielli and I. Filippetti, Selective defoliation affects plant growth, fruit transcriptional ripening program and flavonoid metabolism in grapevine, BMC Plant Biol., 2013, 13, 30 CrossRef CAS PubMed.
- C. Wijewardana, W. B. Henry, W. Gao and K. R. Reddy, Interactive effects on CO2, drought, and ultraviolet-B radiation on maize growth and development, J. Photochem. Photobiol., B., 2016, 160, 198–209 CrossRef CAS PubMed.
- M. Dainese, S. Aikio, P. E. Hulme, A. Bertolli, F. Prosser and L. Marini, Human disturbance and upward expansion of plants in a warming climate, Nat. Clim. Change, 2017, 7, 577–580 CrossRef.
- J. Dolezal, M. Dvorsky, M. Kopecky, P. Liancourt, I. Hiiesalu, M. Macek, J. Altman, Z. Chlumska, K. Rehakova, K. Capkova, J. Borovec, O. Mudrak, J. Wild and F. Schweingruber, Vegetation dynamics at the upper elevational limit of vascular plants in Himalaya, Sci. Rep., 2016, 6, 24881 CrossRef CAS PubMed.
- IPCC, Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part A: Global and Sectoral Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, ed. C. B. Field, V. R. Barros, D. J. Dokken, K. J. Mach, M. D. Mastrandrea, T. E. Bilir, M. Chatterjee, K. L. Ebi, Y. O. Estrada, R. C. Genova, B. Girma, E. S. Kissel, A. N. Levy, S. MacCracken, P. R. Mastrandrea and L. L. White, Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, 2014 Search PubMed.
- M. Blumthaler, W. Ambach and R. Ellinger, Increase in solar UV radiation with altitude, J. Photochem. Photobiol., B., 1997, 39, 130–134 CrossRef CAS.
- M. M. Caldwell, R. Robberecht and W. D. Billings, A steep latitudinal gradient of solar ultraviolet-B radiation in the arctic-alpine life zone, Ecol., 1980, 61, 600–611 CrossRef.
- A. Wolf, N. B. Zimmerman, W. R. L. Anderegg, P. E. Busby and J. Christensen, Altitudinal shifts of the native and introduced flora of California in the context of 20th-century warming, Glob. Ecol. Biogeog., 2016, 25, 418–429 CrossRef.
- C. Körner, The use of ‘altitude’ in ecological research, Trend. Ecol. Evolut., 2007, 22, 569–574 CrossRef PubMed.
- G. Fu and Z.-X. Shen, Effects of enhanced UV-B radiation on plant physiology and growth on the Tibetan Plateau: A meta-analysis, Acta Physiol. Plant, 2017, 39, 85 CrossRef.
- J. C. Mejia-Giraldo, K. Henao-Zuluaga, C. Gallardo, L. Atehortua and M. A. Puertas-Mejia, Novel in vitro antioxidant and photoprotection capacity of plants from high altitude ecosystems of Colombia, Photochem. Photobiol., 2016, 92, 150–157 CrossRef CAS PubMed.
- Y. Zhang, L. Feng, H. Jiang, Y. Zhang and S. Zhang, Different proteome profiles between male and female Populus cathayana exposed to UV-B radiation, Front. Plant Sci., 2017, 8, 320 Search PubMed.
- Q. W. Wang, C. Kamiyama, J. Hidema and K. Hikosaka, Ultraviolet-B-induced DNA damage and ultraviolet-B tolerance mechanisms in species with different functional groups coexisting in subalpine moorlands, Oecologia, 2016, 181, 1069–1082 CrossRef PubMed.
- M. Sun, T. Su, S.-B. Zhang, S.-F. Li, J. Anberree-Lebreton and Z.-K. Zhou, Variations in leaf morphological traits of Quercus guyavifolia (Fagaceae) were mainly influenced by water and ultraviolet irradiation at high elevations on the Qinghai-Tibet Plateau, China, Int. J. Agric. Biol., 2016, 18, 266–273 CrossRef.
- A. Albert, V. Sareedenchai, W. Heller, H. K. Seidlitz and C. Zidorn, Temperature is the key to altitudinal variation of phenolics in Arnica montana L. cv. ARBO, Oecologia, 2009, 160, 1–8 CrossRef PubMed.
- R. G. León-Chan, M. López-Meyer, T. Osuna-Enciso, J. A. Sañudo-Barajas, J. B. Heredia and J. León-Félix, Low temperature and ultraviolet-B radiation affect chlorophyll content and induce the accumulation of UV-B-absorbing and antioxidant compounds in bell pepper (Capsicum annuum) plants, Environ. Exp. Bot., 2017, 139, 143–151 CrossRef.
- H. Kohler, R. A. Contreras, M. Pizarro, R. Cortes-Antiquera and G. E. Zuniga, Antioxidant responses induced by UVB radiation in Deschampsia antarctica Desv, Front. Plant Sci., 2017, 8, 921 CrossRef PubMed.
- M. J. Waterman, A. S. Nugraha, R. Hendra, G. E. Ball, S. A. Robinson and P. A. Keller, Antarctic moss biflavonoids show high antioxidant and ultraviolet-screening activity, J. Nat. Prod., 2017, 80, 2224–2231 CrossRef CAS PubMed.
- Q.-W. Wang, S. Nagano, H. Ozaki, S.-I. Morinaga, J. Hidema and K. Hikosaka, Functional differentiation in UV-B-induced DNA damage and growth inhibition between highland and lowland ecotypes of two Arabidopsis species, Environ. Exp. Bot., 2016, 131, 110–119 CrossRef CAS.
- V. N. Ibañez, F. J. Berli, R. W. Masuelli, R. A. Bottini and C. F. Marfil, Influence of altitude and enhanced ultraviolet-B radiation on tuber production, seed viability, leaf pigments and morphology in the wild potato species Solanum kurtzianum Bitter & Wittm collected from an elevational gradient, Plant Sci., 2017, 261, 60–68 CrossRef PubMed.
- P. W. Barnes, R. J. Ryel and S. D. Flint, UV screening in native and non-native plant species in the tropical alpine: Implications for climate change-driven migration of species to higher elevations, Front. Plant Sci., 2017, 8, 1451 CrossRef PubMed.
- A. M. Davidson, M. Jennions and A. B. Nicotra, Do invasive species show higher phenotypic plasticity than native species and, if so, is it adaptive? A meta-analysis, Ecol. Lett., 2011, 14, 419–431 CrossRef PubMed.
- B. A. Bodhaine, E. G. Dutton, D. J. Hofmann, R. L. McKenzie and P. V. Johnston, UV measurements at Mauna Loa: July 1995 to July 1996, J. Geophys. Res. Atmos., 1997, 102, 19265–19273 CrossRef CAS.
- A. Castagna, K. Csepregi, S. Neugart, G. Zipoli, K. Večeřová, G. Jakab, T. Jug, L. Llorens, J. Martínez-Abaigar, J. Martínez-Lüscher, E. Núñez-Olivera, A. Ranieri, K. Schoedl-Hummel, M. Schreiner, P. Teszlák, S. Tittmann, O. Urban, D. Verdaguer, M. A. K. Jansen and É. Hideg, Environmental plasticity of Pinot noir grapevine leaves; a trans-European study of morphological and biochemical changes along a 1500 km latitudinal climatic gradient, Plant Cell Environ., 2017, 40, 2790–2805 CrossRef CAS PubMed.
- S. I. Semerdjieva, E. Sheffield, G. K. Phoenix, D. GwynnJones, T. V. Callaghan and G. N. Johnson, Contrasting strategies for UV-B screening in sub-Arctic dwarf shrubs, Plant Cell Environ., 2003, 26, 957–964 CrossRef.
- A. G. Roro, M. T. Terfa, K. A. Solhaug, A. Tsegaye, J. E. Olsen and S. Torre, The impact of UV radiation at high altitudes close to the equator on morphology and productivity of pea (Pisum sativum) in different seasons, S. Afr. J. Bot., 2016, 106, 119–128 CrossRef.
- R. Alonso, F. J. Berli, A. Fontana, P. Piccoli and R. Bottini, Malbec grape (Vitis vinifera L.) responses to the environment: Berry phenolics as influenced by solar UV-B, water deficit and sprayed abscisic acid, Plant Physiol. Biochem., 2016, 109, 84–90 CrossRef CAS PubMed.
- F. M. Dillon, H. D. Chludil and J. A. Zavala, Solar UV-B radiation modulates chemical defenses against Anticarsia gemmatalis larvae in leaves of field-grown soybean, Phytochemistry, 2017, 141, 27–36 CrossRef CAS PubMed.
- A. Suthaparan, K. A. Solhaug, A. Stensvand and H. R. Gislerød, Determination of UV action spectra affecting the infection process of Oidium neolycopersici, the cause of tomato powdery mildew, J. Photochem. Photobiol., B., 2016, 156, 41–49 CrossRef CAS PubMed.
- H. Wang, M. Gui, X. Tian, X. Xin, T. Wang and J. li, Effects of UV-B on vitamin C, phenolics, flavonoids and their related enzyme activities in mung bean sprouts (Vigna radiata), Int. J. Food Sci. Technol., 2017, 52, 827–833 CrossRef CAS.
- K. K. Choudhary and S. B. Agrawal, Assessment of fatty acid profile and seed mineral nutrients of two soybean (Glycine max L.) cultivars under elevated ultraviolet-B: Role of ROS, pigments and antioxidants, Photochem. Photobiol., 2016, 92, 134–143 CrossRef CAS PubMed.
- K. R. Reddy, H. Patro, S. Lokhande, N. Bellaloui and W. Gao, Ultraviolet-B radiation alters soybean growth and seed quality, Food Nutr. Sci., 2016, 7, 55–66 CrossRef CAS.
- R. Tripathi and S. B. Agrawal, Effect of supplemental UV-B on yield, seed quality, oil content and fatty acid composition of Brassica campestris L. under natural field conditions, Qual. Assur. Saf. Crops Foods, 2016, 8, 11–20 CrossRef CAS.
- C. T. T. Nguyen, S. Lim, J. G. Lee and E. J. Lee, VcBBX, VcMYB21, and VcR2R3MYB transcription factors are involved in UV-B-induced anthocyanin biosynthesis in the peel of harvested blueberry fruit, J. Agric. Food Chem., 2017, 65, 2066–2073 CrossRef CAS PubMed.
- G. Wu, J. F. Bornman, S. J. Bennett, M. W. Clarke, Z. Fang and S. K. Johnson, Individual polyphenolic profiles and antioxidant activity in sorghum grains are influenced by very low and high solar UV radiation and genotype, J. Cereal Sci., 2017, 77, 17–23 CrossRef CAS.
- L. Dykes and L. Rooney, Phenolic compounds in cereal grains and their health benefits, Cereal Foods World, 2007, 52, 105–111 CAS.
- J. D. Wightman and R. A. Heuberger, Effect of grape and other berries on cardiovascular health, J. Sci. Food Agric., 2015, 95, 1584–1597 CrossRef CAS PubMed.
- A. Umeno, M. Horie, K. Murotomi, Y. Nakajima and Y. Yoshida, Antioxidative and antidiabetic effects of natural polyphenols and isoflavones, Molecules, 2016, 21, 708 CrossRef PubMed.
- Z. Rasines-Perea and P.-L. Teissedre, Grape polyphenols’ effects in human cardiovascular diseases and diabetes, Molecules, 2017, 22, 68 CrossRef PubMed.
- A. Luengo Escobar, F. Magnum de Oliveira Silva, P. Acevedo, A. Nunes-Nesi, M. Alberdi and M. Reyes-Díaz, Different levels of UV-B resistance in Vaccinium corymbosum cultivars reveal distinct backgrounds of phenylpropanoid metabolites, Plant Physiol. Biochem., 2017, 118, 541–550 CrossRef CAS PubMed.
- C. Inostroza-Blancheteau, P. Acevedo, R. Loyola, P. Arce-Johnson, M. Alberdi and M. Reyes-Díaz, Short-term UV-B radiation affects photosynthetic performance and antioxidant gene expression in highbush blueberry leaves, Plant Physiol. Biochem., 2016, 107, 301–309 CrossRef CAS PubMed.
- M. M. Caldwell and S. D. Flint, Stratospheric ozone reduction, solar UV-B radiation and terrestrial ecosystems, Clim. Change, 1994, 28, 375–394 CrossRef CAS.
- C. L. Ballaré, Light regulation of plant defense, Annu. Rev. Plant Biol., 2014, 65, 335–363 CrossRef PubMed.
- R. Escobar-Bravo, P. G. L. Klinkhamer and K. A. Leiss, Interactive effects of UV-B light with abiotic factors on plant growth and chemistry, and their consequencesfor defense against arthropod herbivores, Front. Plant Sci., 2017, 8, 278 Search PubMed.
- J. A. Zavala, C. A. Mazza, F. M. Dillon, H. D. Chludil and C. L. Ballaré, Soybean resistance to stink bugs (Nezara viridula and Piezodorus guildinii) increases with exposure to solar UV-B radiation and correlates with isoflavonoid content in pods under field conditions, Plant Cell Environ., 2015, 38, 920–928 CrossRef CAS PubMed.
- C. L. Ballaré, C. A. Mazza, A. T. Austin and R. Pierik, Canopy light and plant health, Plant Physiol., 2012, 160, 145–155 CrossRef PubMed.
- I. Major, M. Campos and J. Moreno, The role of specialized photoreceptors in the protection of energy-rich tissues, Agronomy, 2017, 7, 23 CrossRef.
- O. I. L. Mawphlang and E. V. Kharshiing, Photoreceptor mediated plant growth responses: Implications for photoreceptor engineering toward improved performance in crops, Front. Plant Sci., 2017, 8, 1181 CrossRef PubMed.
- A. T. Austin, M. S. Méndez and C. L. Ballaré, Photodegradation alleviates the lignin bottleneck for carbon turnover in terrestrial ecosystems, Proc. Natl. Acad. Sci. U. S. A., 2016, 113, 4392–4397 CrossRef CAS PubMed.
- P. W. Barnes, H. L. Throop, S. R. Archer, D. D. Breshears, R. L. McCulley and M. A. Tobler, Sunlight and soil-litter mixing: drivers of litter decomposition in drylands, Prog. Bot., 2015, 76, 273–302 Search PubMed.
- J. Y. King, L. A. Brandt and E. C. Adair, Shedding light on plant litter decomposition: advances, implications and new directions in understanding the role of photodegradation, Biogeochemistry, 2012, 111, 57–81 CrossRef.
- A. T. Austin and C. L. Ballaré, Dual role of lignin in plant litter decomposition in terrestrial ecosystems, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 4618–4622 CrossRef CAS PubMed.
- Y. Lin, R. D. Scarlett and J. Y. King, Effects of UV photodegradation on subsequent microbial decomposition of Bromus diandrus litter, Plant Soil, 2015, 395, 263–271 CrossRef CAS.
- E. C. Adair, W. J. Parton, J. Y. King, L. A. Brandt and Y. Lin, Accounting for photodegradation dramatically improves prediction of carbon losses in dryland systems, Ecosphere, 2017, 8, e01892 CrossRef.
- X. Pan, Y.-B. Song, G.-F. Liu, Y.-K. Hu, X.-H. Ye, W. K. Cornwell, A. Prinzing, M. Dong and J. H. C. Cornelissen, Functional traits drive the contribution of solar radiation to leaf litter decomposition among multiple arid-zone species, Sci. Rep., 2015, 5, 13217 CrossRef CAS PubMed.
- D. B. Hewins, R. L. Sinsabaugh, S. R. Archer and H. L. Throop, Soil–litter mixing and microbial activity mediate decomposition and soil aggregate formation in a sandy shrub-invaded Chihuahuan Desert grassland, Plant Ecol., 2017, 218, 459–474 CrossRef.
- J. Wang, L. Liu, X. Wang, S. Yang, B. Zhang, P. Li, C. Qiao, M. Deng and W. Liu, High night-time humidity and dissolved organic carbon content support rapid decomposition of standing litter in a semi-arid landscape, Funct. Ecol., 2017, 31, 1659–1668 CrossRef.
- J. Wang, S. Yang, B. Zhang, W. Liu, M. Deng, S. Chen and L. Liu, Temporal dynamics of ultraviolet radiation impacts on litter decomposition in a semi-arid ecosystem, Plant Soil, 2017, 419, 71–81 CrossRef CAS.
- G. Huang, H.-m. Zhao and Y. Li, Litter decomposition in hyper-arid deserts: Photodegradation is still important, Sci. Total Environ., 2017, 601, 784–792 CrossRef PubMed.
- M. Almagro, J. Martínez-López, F. T. Maestre and A. Rey, The contribution of photodegradation to litter decomposition in semiarid Mediterranean grasslands depends on its interaction with local humidity conditions, litter quality and position, Ecosystems, 2017, 20, 527–542 CrossRef CAS.
- D. Gliksman, A. Rey, R. Seligmann, R. Dumbur, O. Sperling, Y. Navon, S. Haenel, P. De Angelis, J. A. Arnone and J. M. Grünzweig, Biotic degradation at night, abiotic degradation at day: positive feedbacks on litter decomposition in drylands, Glob. Change Biol., 2016, 23, 1564–1574 CrossRef PubMed.
- Z. Ma, W. Yang, F. Wu and B. Tan, Effects of light intensity on litter decomposition in a subtropical region, Ecosphere, 2017, 8, e01770 CrossRef.
- P. I. Araujo and A. T. Austin, A shady business: pine afforestation alters the primary controls on litter decomposition along a precipitation gradient in Patagonia, Argentina, J. Ecol., 2015, 103, 1408–1420 CrossRef CAS.
- D. B. Hewins and H. L. Throop, Leaf litter decomposition is rapidly enhanced by the co-occurrence of monsoon rainfall and soil-litter mixing across a gradient of coppice dune development in the Chihuahuan Desert, J. Arid Environ., 2016, 129, 111–118 CrossRef.
- T. Bosco, M. B. Bertiller and A. L. Carrera, Combined effects of litter features, UV radiation, and soil water on litter decomposition in denuded areas of the arid Patagonian Monte, Plant Soil, 2016, 406, 71–82 CrossRef CAS.
- M. Almagro, F. T. Maestre, J. Martínez-López, E. Valencia and A. Rey, Climate change may reduce litter decomposition while enhancing the contribution of photodegradation in dry perennial Mediterranean grasslands, Soil Biol. Biochem., 2015, 90, 214–223 CrossRef CAS.
- M. Chen, W. J. Parton, E. C. Adair, S. Asao, M. D. Hartman and W. Gao, Simulation of the effects of photodecay on long-term litter decay using DayCent, Ecosphere, 2016, 7, e01631 CrossRef.
- G. Myhre, D. Shindell, F. M. Bréon, W. Collins, J. Fuglestvedt, J. Huang, D. Koch, J. F. Lamarque, D. Lee, B. Mendoza, T. Nakajima, A. Robock, G. Stephens, T. Takemura and H. Zhang, Anthropogenic and natural radiative forcing, in Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, ed. T. F. Stocker, D. Qin, G.-K. Plattner, M. Tignor, S. K. Allen, J. Boschung, A. Nauels, Y. Xia, V. Bex and P. M. Midgley, Cambridge University Press, Cambridge and New York, 2013 Search PubMed.
- EPA, Understanding global warming potentials, United States Environmental Protection Agency, https://www.epa.gov/ghgemissions/understanding-global-warming-potentials, accessed September 9, 2017 Search PubMed.
- T. N. Mikkelsen, D. Bruhn and P. Ambus, Solar UV irradiation-induced production of greenhouse gases from plant surfaces: From leaf to Earth, Prog. Bot., 2016, 78, 407–437 Search PubMed.
- D. Bruhn, T. N. Mikkelsen, M. M. M. Rolsted, H. Egsgaard and P. Ambus, Leaf surface wax is a source of plant methane formation under UV radiation and in the presence of oxygen, Plant Biol., 2014, 16, 512–516 CrossRef CAS PubMed.
- I. Vigano, H. van Weelden, R. Holzinger, F. Keppler, A. McLeod and T. Rockmann, Effect of UV radiation and temperature on the emission of methane from plant biomass and structural components, Biogeoscience, 2008, 5, 937–947 CrossRef CAS.
- F. Keppler, J. T. G. Hamilton, M. Brass and T. Rockmann, Methane emissions from terrestrial plants under aerobic conditions, Nature, 2006, 439, 187–191 CrossRef CAS PubMed.
- A. A. Bloom, J. Lee-Taylor, S. Madronich, D. J. Messenger, P. I. Palmer, D. S. Reay and A. R. McLeod, Global methane emission estimates from ultraviolet irradiation of terrestrial plant foliage, New Phytol., 2010, 187, 417–425 CrossRef CAS PubMed.
- A. M. Abdulmajeed and M. M. Qaderi, Intrashoot variation in aerobic methane emissions from pea plants exposed to multiple abiotic stresses, Acta Physiol. Plant., 2017, 39, 124 CrossRef.
- A. M. Abdulmajeed, S. R. Derby, S. K. Strickland and M. M. Qaderi, Interactive effects of temperature and UVB radiation on methane emissions from different organs of pea plants grown in hydroponic system, J. Photochem. Photobiol., B., 2017, 166, 193–201 CrossRef CAS PubMed.
- W. T. Fraser, E. Blei, S. C. Fry, M. F. Newman, D. S. Reay, K. A. Smith and A. R. McLeod, Emission of methane, carbon monoxide, carbon dioxide and short-chain hydrocarbons from vegetation foliage under ultraviolet irradiation, Plant Cell Environ., 2015, 38, 980–989 CrossRef CAS PubMed.
- D. J. Erickson, B. Sulzberger, R. G. Zepp and A. T. Austin, Effects of stratospheric ozone depletion, solar UV radiation, and climate change on biogeochemical cycling: interactions and feedbacks, Photochem. Photobiol. Sci., 2015, 14, 127–148 CAS.
- D. Bruhn, K. R. Albert, T. N. Mikkelsen and P. Ambus, UV-induced N2O emission from plants, Atmos. Environ., 2014, 99, 206–214 CrossRef CAS.
- K. Butterbach-Bahl, E. M. Baggs, M. Dannenmann, R. Kiese and S. Zechmeister-Boltenstern, Nitrous oxide emissions from soils: how well do we understand the processes and their controls?, Philos. Trans. R. Soc., B., 2013, 368, 20130122 CrossRef PubMed.
- P. Porada, U. Pöschl, A. Kleidon, C. Beer and B. Weber, Estimating global nitrous oxide emissions by lichens and bryophytes with a process-based productivity model, Biogeoscience, 2017, 14, 1593–1602 CrossRef.
- K. Lenhart, B. Weber, W. Elbert, J. Steinkamp, T. Clough, P. Crutzen, U. Pöschl and F. Keppler, Nitrous oxide and methane emissions from cryptogamic covers, Glob. Change Biol., 2015, 21, 3889–3900 CrossRef PubMed.
- Q. Zhuang, Y. Lu and M. Chen, An inventory of global N2O emissions from the soils of natural terrestrial ecosystems, Atmos. Environ., 2012, 47, 66–75 CrossRef CAS.
- S. Solomon, D. Qin, M. Manning, Z. Chen, M. Marquis, K. B. Averyt, M. Tignor and H. L. Miller, Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change, Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, 2007 Search PubMed.
- D. Bruhn, I. M. Møller, T. N. Mikkelsen and P. Ambus, Terrestrial plant methane production and emission, Physiol. Plant., 2012, 144, 201–209 CrossRef CAS PubMed.
- K. J. Dinsmore, J. Drewer, P. E. Levy, C. George, A. Lohila, M. Aurela and U. M. Skiba, Growing season CH4 and N2O fluxes from a subarctic landscape in northern Finland; from chamber to landscape scale, Biogeoscience, 2017, 14, 799–815 CrossRef.
- F. Savi, C. Di Bene, L. Canfora, C. Mondini and S. Fares, Environmental and biological controls on CH4 exchange over an evergreen Mediterranean forest, Agric. Forest Meteorol., 2016, 226–227, 67–79 CrossRef.
- A. B. Martel and M. M. Qaderi, Light quality and quantity regulate aerobic methane emissions from plants, Physiologia Planatarum, 2017, 159, 313–328 CrossRef CAS PubMed.
- J. Liu, H. Chen, Q. Zhu, Y. Shen, X. Wang, M. Wang and C. Peng, A novel pathway of direct methane production and emission by eukaryotes including plants, animals and fungi: An overview, Atmos. Environ., 2015, 115, 26–35 CrossRef CAS.
- M. J. Carmichael, E. S. Bernhardt, S. L. Bräuer and W. K. Smith, The role of vegetation in methane flux to the atmosphere: should vegetation be included as a distinct category in the global methane budget?, Biogeochemistry, 2014, 119, 1–24 CrossRef CAS.
- L. Rizzini, J. J. Favory, C. Cloix, D. Faggionato, A. O'Hara, E. Kaiserli, R. Baumeister, E. Schäfer, F. Nagy, G. I. Jenkins and R. Ulm, Perception of UV-B by the Arabidopsis UVR8 protein, Science, 2011, 332, 103–106 CrossRef CAS PubMed.
- J. M. Christie, A. S. Arvai, K. J. Baxter, M. Heilmann, A. J. Pratt, A. O'Hara, S. M. Kelly, M. Hothorn, B. O. Smith, K. Hitomi, G. I. Jenkins and E. D. Getzoff, Plant UVR8 photoreceptor senses UV-B by tryptophan-mediated disruption of cross-dimer salt bridges, Science, 2012, 335, 1492–1496 CrossRef CAS PubMed.
- D. Wu, Q. Hu, Z. Yan, W. Chen, C. Y. Yan, X. Huang, J. Zhang, P. Y. Yang, H. T. Deng, J. W. Wang, X. W. Deng and Y. G. Shi, Structural basis of ultraviolet-B perception by UVR8, Nature, 2012, 484, 214–219 CrossRef PubMed.
- R. Yin and R. Ulm, How plants cope with UV-B: from perception to response, Curr. Opin. Plant Biol., 2017, 37, 42–48 CrossRef CAS PubMed.
- G. I. Jenkins, Photomorphogenic responses to ultraviolet-B light, Plant Cell Environ., 2017, 40, 2544–2557 CrossRef CAS PubMed.
- M. B. Fernández, V. Tossi, L. Lamattina and R. Cassia, A comprehensive phylogeny reveals functional conservation of the UV-B photoreceptor UVR8 from green algae to higher plants, Front. Plant Sci., 2016, 7, 1698 Search PubMed.
- K. Tilbrook, M. Dubois, C. D. Crocco, R. Yin, R. Chappuis, G. Allorent, E. Schmid-Siegert, M. Goldschmidt-Clermont and R. Ulm, UV-B perception and acclimation in Chlamydomonas reinhardtii, Plant Cell, 2016, 28, 966–983 CAS.
- G. Soriano, C. Cloix, M. Heilmann, E. Núñez-Olivera, J. Martínez-Abaigar and G. I. Jenkins, Evolutionary conservation of structure and function of the UVR8 photoreceptor from the liverwort Marchantia polymorpha and the moss Physcomitrella patens, New Phytol., 2017, 161, 151–162 Search PubMed.
- S. Hayes, C. N. Velanis, G. I. Jenkins and K. A. Franklin, UV-B detected by the UVR8 photoreceptor antagonizes auxin signaling and plant shade avoidance, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 11894–11899 CrossRef CAS PubMed.
- S. Hayes, A. Sharma, D. P. Fraser, M. Trevisan, C. K. Cragg-Barber, E. Tavridou, C. Fankhauser, G. I. Jenkins and K. A. Franklin, UV-B perceived by the UVR8 photoreceptor inhibits plant thermomorphogenesis, Curr. Biol., 2017, 27, 120–127 CrossRef CAS PubMed.
- C. A. Mazza and C. L. Ballaré, Photoreceptors UVR8 and phytochrome B cooperate to optimize plant growth and defense in patchy canopies, New Phytol., 2015, 207, 4–9 CrossRef PubMed.
- K. M. W. Findlay and G. I. Jenkins, Regulation of UVR8 photoreceptor dimer/monomer photo-equilibrium in Arabidopsis plants grown under photoperiodic conditions, Plant Cell Environ., 2016, 39, 1706–1714 CrossRef CAS PubMed.
- C. L. Ballaré and R. Pierik, The shade-avoidance syndrome: multiple signals and ecological consequences, Plant Cell Environ., 2017, 40, 2530–2543 CrossRef PubMed.
- L. O. Morales, M. Brosché, J. Vainonen, G. I. Jenkins, J. J. Wargent, N. Sipari, A. Strid, A. V. Lindfors, R. Tegelberg and P. J. Aphalo, Multiple roles for UV RESISTANCE LOCUS8 in regulating gene expression and metabolite accumulation in Arabidopsis under solar ultraviolet radiation, Plant Physiol., 2013, 161, 744–759 CrossRef CAS PubMed.
- A. Coffey, E. Prinsen, M. A. K. Jansen and J. Conway, The UVB photoreceptor UVR8 mediates accumulation of UV-absorbing pigments, but not changes in plant morphology, under outdoor conditions, Plant Cell Environ., 2017, 40, 2250–2260 CrossRef CAS PubMed.
- P. V. Demkura and C. L. Ballaré, UVR8 mediates UV-B-induced Arabidopsis defense responses against Botrytis cinerea by controlling sinapate accumulation, Mol. Plant, 2012, 5, 642–652 CrossRef PubMed.
- J. Rozema, B. van Geel, L. O. Bjorn, J. Lean and S. Madronich, Paleoclimate. Toward solving the UV puzzle, Science, 2002, 296, 1621–1622 CrossRef CAS PubMed.
- W. T. Fraser, B. H. Lomax, P. E. Jardine, W. D. Gosling and M. A. Sephton, Pollen and spores as a passive monitor of ultraviolet radiation, Front. Ecol. Evolut., 2014, 2, 00012 Search PubMed.
- P. E. Jardine, F. A. J. Abernethy, B. H. Lomax, W. D. Gosling and W. T. Fraser, Shedding light on sporopollenin chemistry, with reference to UV reconstructions, Rev. Palaeobot. Palynol., 2017, 238, 1–6 CrossRef.
- A. W. R. Seddon, M. Jokerud, T. Barth, H. J. B. Birks, L. C. Krüger, V. Vandvik and K. J. Willis, Improved quantification of UV-B absorbing compounds in Pinus sylvestris L. pollen grains using an internal standard methodology, Rev. Palaeobot. Palynol., 2017, 247, 97–104 CrossRef.
- B. C. Thomas, B. D. Goracke and S. M. Dalton, Atmospheric constituents and surface-level UVB: Implications for a paleoaltimetry proxy and attempts to reconstruct UV exposure during volcanic episodes, Earth Planet Sci. Lett., 2016, 453, 141–151 CrossRef CAS.
- R. L. McKenzie, P. J. Aucamp, A. F. Bais, L. O. Björn, M. Ilyas and S. Madronich, Ozone depletion and climate change: impacts on UV radiation, Photochem. Photobiol. Sci., 2011, 10, 182–198 CAS.
- M. Bağcıoğlu, A. Kohler, S. Seifert, J. Kneipp, B. Zimmermann and S. McMahon, Monitoring of plant-environment interactions by high-throughput FTIR spectroscopy of pollen, Methods Ecol. Evolut., 2017, 8, 870–880 CrossRef.
- P. E. Jardine, W. T. Fraser, B. H. Lomax, M. A. Sephton, T. M. Shanahan, C. S. Miller and W. D. Gosling, Pollen and spores as biological recorders of past ultraviolet irradiance, Sci. Rep., 2016, 6, 39269 CrossRef CAS PubMed.
- J. J. Wargent, Turning UV-B photobiology into commercial reality, in UV-B Radiation and Plant Life: Molecular Biology to Ecology, ed. B. R. Jordan, CABI International, Oxford, UK, 2017, pp. 162–176 Search PubMed.
- J. Rass, T. Kolbe, N. Lobo-Ploch, T. Wernicke, F. Mehnke, C. Kuhn, J. Enslin, M. Guttmann, C. Reich, A. Mogilatenko, J. Glaab, C. Stoelmacker, M. Lapeyrade, S. Einfeldt, M. Weyers and M. Kneissl, High-power UV-B LEDs with long lifetime, Proc. SPIE, 2015, 9363, 93631K CrossRef.
- M. Kneissl, A brief review of III-Nitride UV emitter technologies and their applications, in III-Nitride Ultraviolet Emitters: Technology and Applications, ed. M. Kneissl and J. Rass, Springer International Publishing, Cham, 2016, pp. 1–25 Search PubMed.
- P. J. Aphalo, A. Albert, L. O. Björn, A. McLeod, T. M. Robson and E. Rosenqvist, Beyond the visible: A handbook of best practice in plant UV photobiology, in COST Action FA0906 UV4growth, University of Helsinki, Department of Biosciences, Division of Plant Biology Helsinki, Finland, http://hdl.handle.net/10138/37558, 2012, p. 176 Search PubMed.
- P. J. Aphalo, The r4photobiology suite: Spectral irradiance, UV4 Plants Bull., 2015, 2015, 21–29 Search PubMed.
- P. J. Aphalo, Quantification of UV radiation, in UV-B Radiation and Plant Life: Molecular Biology to Ecology, ed. B. R. Jordan, CABI International, Oxford, UK, 2017, pp. 10–22 Search PubMed.
- P. J. Aphalo, T. M. Robson and J. Piiparinen, How to check an array spectrometer, Int. Assoc. Plant UV Res. Search PubMed , http://uv4plants.org/methods/how-to-check-an-array-spectrometer/, updated June 2, 2013, accessed November 11, 2017.
- L. Wainwright, A. V. Parisi and N. Downs, Dosimeter based on 8-methoxypsoralen for UVA exposures over extended periods, J. Photochem. Photobiol., B., 2015, 148, 246–251 CrossRef CAS PubMed.
- A. Amar and A. V. Parisi, Optical properties of a long dynamic range chemical UV dosimeter based on solvent cast polyvinyl chloride (PVC), J. Photochem. Photobiol., B., 2013, 128, 92–99 CrossRef CAS PubMed.
- A. V. Parisi, A. Amar and D. P. Igoe, Long-term UV dosimeter based on polyvinyl chloride for plant damage effective UV exposure measurements, Agric. Forest Meteorol., 2017, 243, 68–73 CrossRef.
- H. Araki, J. Kim, S. Zhang, A. Banks, K. E. Crawford, X. Sheng, P. Gutruf, Y. Shi, R. M. Pielak and J. A. Rogers, Materials and device designs for an epidermal UV colorimetric dosimeter with near field communication capabilities, Adv. Funct. Mater., 2017, 27, 1604465 CrossRef.
- J. C. Comiso, W. N. Meier and R. A. Gersten, Variability and trends in the Arctic Sea ice cover: Results from different techniques, J. Geophys. Res. Oceans, 2017, 122, 6883–6900 Search PubMed.
- M. J. Behrenfeld, R. T. O'Malley, D. A. Siegel, C. R. McClain, J. L. Sarmiento, G. C. Feldman, A. J. Milligan, P. G. Falkowski, R. M. Letelier and E. S. Boss, Climate-driven trends in contemporary ocean productivity, Nature, 2006, 444, 752–755 CrossRef CAS PubMed.
- B. M. Kraemer, O. Anneville, S. Chandra, M. Dix, E. Kuusisto, D. M. Livingstone, A. Rimmer, G. Schladow, E. A. Silow, L. M. Sitoki, R. Tamatamah, Y. Vadeboncoeur and P. B. McIntyre, Morphometry and average temperature affect lake stratification responses to climate change, Geophys. Res. Lett., 2015, 42, 4981–4988 CrossRef.
- R. Somavilla, C. González-Pola and J. Fernández-Diaz, The warmer the ocean surface, the shallower the mixed layer. How much of this is true?, J. Geophys. Res. Oceans, 2017, 122, 7698–7716 CAS.
- C. E. Williamson, S. Madronich, A. Lal, R. G. Zepp, R. M. Lucas, E. P. Overholt, K. C. Rose, G. Schladow and J. Lee-Taylor, Climate change-induced increases in precipitation are reducing the potential for solar ultraviolet radiation to inactivate pathogens in surface waters, Sci. Rep., 2017, 7, 13033 CrossRef PubMed.
- J. G. Molinos, M. T. Burrows and E. S. Poloczanska, Ocean currents modify the coupling between climate change and biogeographical shifts, Sci. Rep., 2017, 7, 1332 CrossRef PubMed.
- M. Feng, M. J. McPhaden, S. P. Xie and J. Hafner, La Nina forces unprecedented Leeuwin Current warming in 2011, Sci. Rep., 2013, 3, 1277 CrossRef CAS PubMed.
- M. R. Garcia-Huidobro, M. Aldana, C. Duarte, C. Galban-Malagon and J. Pulgar, Seawater-temperature and UV-radiation interaction modifies oxygen consumption, digestive process and growth of an intertidal fish, Mar. Environ. Res., 2017, 129, 408–412 CrossRef CAS PubMed.
- F. M. Monteiro, L. T. Bach, C. Brownlee, P. Bown, R. E. M. Rickaby, A. J. Poulton, T. Tyrrell, L. Beaufort, S. Dutkiewicz, S. Gibbs, M. A. Gutowska, R. Lee, U. Riebesell, J. Young and A. Ridgwell, Why marine phytoplankton calcify, Sci. Adv., 2016, 2, e1501822 Search PubMed.
- C. E. Cornwall, S. Comeau and M. T. McCulloch, Coralline algae elevate pH at the site of calcification under ocean acidification, Glob. Change Biol., 2017, 33, 4245–4256 CrossRef PubMed.
- P. Jin, J. C. Ding, T. Xing, U. Riebesell and K. S. Gao, High levels of solar radiation offset impacts of ocean acidification on calcifying and non-calcifying strains of Emiliania huxleyi, Mar. Ecol.: Prog. Ser., 2017, 568, 47–58 CrossRef.
- F. J. L. Gordillo, R. Carmona, B. Vinegla, C. Wiencke and C. Jimenez, Effects of simultaneous increase in temperature and ocean acidification on biochemical composition and photosynthetic performance of common macroalgae from Kongsfjorden (Svalbard), Polar Biol., 2016, 39, 1993–2007 CrossRef.
- D. Häder and K. Gao, The Impacts of Climate Change on Marine Phytoplankton, in Climate Change Impacts on Fisheries and Aquaculture: A Global Analysis, ed. B. F. Phillips and M. Perez-Ramirez, Wiley, Hoboken, NJ, 2017, ch. 27 Search PubMed.
- A. C. Dave, Correction to “Examining the global record of interannual variability in stratification and marine productivity in the low-and mid-latitude ocean”, J. Geophys. Res. Oceans, 2014, 119, 2121–2128 Search PubMed.
- A. C. Dave and M. S. Lozier, Examining the global record of interannual variability in stratification and marine productivity in the low-latitude and mid-latitude ocean, J. Geophys. Res. Oceans, 2013, 118, 3114–3127 Search PubMed.
- W. Li, Y. L. Yang, Z. Z. Li, J. T. Xu and K. S. Gao, Effects of seawater acidification on the growth rates of the diatom Thalassiosira, (Conticribra) weissflogii under different nutrient, light, and UV radiation regimes, J. Appl. Phycol., 2017, 29, 133–142 CrossRef CAS.
- L. Wolinski, B. Modenutti, M. S. Souza and E. Balseiro, Interactive effects of temperature, ultraviolet radiation and food quality on zooplankton alkaline phosphatase activity, Environ. Pollut., 2016, 213, 135–142 CrossRef CAS PubMed.
- S. Peng, H. Liao, T. Zhou and S. Peng, Effects of UVB radiation on freshwater biota: A meta-analysis, Glob. Ecol. Biogeog., 2016, 26, 500–510 CrossRef.
- E. G. Kazerouni, C. E. Franklin and F. Seebacher, Parental exposure modulates the effects of UV-B on offspring in guppies, Funct. Ecol., 2017, 31, 1082–1090 CrossRef.
- M. Llabrés, S. Agustí, M. Fernández, A. Canepa, F. Maurin, F. Vidal and C. M. Duarte, Impact of elevated UVB radiation on marine biota: a meta-analysis, Glob. Ecol. Biogeog., 2013, 22, 131–144 CrossRef.
- C. T. Solomon, S. E. Jones, B. C. Weidel, I. Buffam, M. L. Fork, J. Karlsson, S. Larsen, J. T. Lennon, J. S. Read, S. Sadro and J. E. Saros, Ecosystem consequences of changing inputs of terrestrial dissolved organic matter to lakes: Current knowledge and future challenges, Ecosystems, 2015, 18, 376–389 CrossRef.
- H. A. de Wit, S. Valinia, G. A. Weyhenmeyer, M. N. Futter, P. Kortelainen, K. Austnes, D. O. Hessen, A. Raike, H. Laudon and J. Vuorenmaa, Current browning of surface waters will be further promoted by wetter climate, Environ. Sci. Technol. Lett., 2016, 3, 430–435 CrossRef CAS.
- B. Sulzberger and J. S. Arey, Impacts of polar changes on the UV-induced mineralization of terrigenous dissolved organic matter, Environ. Sci. Technol., 2016, 50, 6621–6631 CrossRef CAS PubMed.
- C. E. Williamson, E. P. Overholt, J. A. Brentrup, R. M. Pilla, T. H. Leach, S. G. Schladow, J. D. Warren, S. S. Urmy, S. Sadro, S. Chandra and P. J. Neale, Sentinel responses to droughts, wildfires, and floods: effects of UV radiation on lakes and their ecosystem services, Front. Ecol. Environ., 2016, 14, 102–109 CrossRef.
- R. Wolf, T. Andersen, D. O. Hessen and K. Hylland, The influence of dissolved organic carbon and ultraviolet radiation on the genomic integrity of Daphnia magna, Funct. Ecol., 2017, 31, 848–855 CrossRef.
- M. Lindholm, R. Wolf, A. Finstad and D. O. Hessen, Water browning mediates predatory decimation of the Arctic fairy shrimp Branchinecta paludosa, Freshwater Biol., 2016, 61, 340–347 CrossRef.
- R. L. Smyth, C. Sobrino, J. Phillips-Kress, H. C. Kim and P. J. Neale, Phytoplankton photosynthetic response to solar ultraviolet irradiance in the Ross Sea Polynya: Development and evaluation of a time-dependent model with limited repair, Limnol. Oceanogr., 2012, 57, 1602–1618 CrossRef.
- R. L. Smyth, P. J. Neale, C. Akan and A. E. Tejada-Martinez, Quantifying phytoplankton productivity and photoinhibition in the Ross Sea Polynya with large eddy simulation of Langmuir circulation, J. Geophys. Res. Oceans, 2017, 122, 5545–5565 CAS.
- I. N. Flamarique, Diminished foraging performance of a mutant zebrafish with reduced population of ultraviolet cones, Proc. R. Soc. B, 2016, 283(1826), 20160058 CrossRef PubMed.
- M. S. Valiñas and E. W. Helbling, Metabolic and behavioral responses of the reef fish Patagonotothen cornucola to ultraviolet radiation: influence of the diet, J. Exp. Mar. Biol. Ecol., 2016, 474, 180–184 CrossRef.
- L. A. Hansson, G. Bianco, M. T. Ekvall, J. Heuschele, S. Hylander and X. Yang, Instantaneous threat escape and differentiated refuge demand among zooplankton taxa, Ecol., 2016, 97, 279–285 CrossRef PubMed.
- S. Urmy, C. E. Williamson, T. H. Leach, S. G. Schladow, E. Overholt and J. D. Warren, Vertical redistribution of zooplankton in an oligotrophic lake associated with reduction in ultraviolet radiation by wildfire smoke, Geophys. Res. Lett., 2016, 43, 3746–3753 CrossRef.
- A. Galletti, S. Seo, S. H. Joo, C. Su and P. Blackwelder, Effects of titanium dioxide nanoparticles derived from consumer products on the marine diatom Thalassiosira pseudonana, Environ. Sci. Pollut. Res., 2016, 23, 21113–21122 CrossRef CAS PubMed.
- M. Sendra, D. Sanchez-Quiles, J. Blasco, I. Moreno-Garrido, L. M. Lubian, S. Perez-Garcia and A. Tovar-Sanchez, Effects of TiO2 nanoparticles and sunscreens on coastal marine microalgae: Ultraviolet radiation is key variable for toxicity assessment, Environ. Int., 2017, 98, 62–68 CrossRef CAS PubMed.
- C. A. Downs, E. Kramarsky-Winter, R. Segal, J. Fauth, S. Knutson, O. Bronstein, F. R. Ciner, R. Jeger, Y. Lichtenfeld, C. M. Woodley, P. Pennington, K. Cadenas, A. Kushmaro and Y. Loya, Toxicopathological effects of the sunscreen UV filter, Oxybenzone (Benzophenone-3), on coral planulae and cultured primary cells and its environmental contamination in Hawaii and the U.S. Virgin Islands, Arch. Environ. Contam. Toxicol., 2016, 70.2, 265–288 CrossRef PubMed.
- M. M. P. Tsui, J. C. W. Lam, T. Y. Ng, P. O. Ang, M. B. Murphy and P. K. S. Lam, Occurrence, distribution, and fate of organic UV filters in coral communities, Environ. Sci. Technol., 2017, 51, 4182–4190 CrossRef CAS PubMed.
- C. Corinaldesi, E. Damiani, F. Marcellini, C. Falugi, L. Tiano, F. Bruge and R. Danovaro, Sunscreen products impair the early developmental stages of the sea urchin Paracentrotus lividus, Sci. Rep., 2017, 7, 7815 CrossRef PubMed.
- H. C. H. Fong, J. C. H. Ho, A. H. Y. Cheung, K. P. Lai and W. K. F. Tse, Developmental toxicity of the common UV filter, benzophenone-2, in zebrafish embryos, Chemosphere, 2016, 164, 413–420 CrossRef CAS PubMed.
- I. Ozaez, M. Aquilino, G. Morcillo and J. L. Martinez-Guitarte, UV filters induce transcriptional changes of different hormonal receptors in Chironomus riparius embryos and larvae, Environ. Pollut., 2016, 214, 239–247 CrossRef CAS PubMed.
- D. Campos, C. Gravato, C. Quintaneiro, O. Golovko, V. Zlabek, A. Soares and J. L. T. Pestana, Toxicity of organic UV-filters to the aquatic midge Chironomus riparius, Ecotoxicol. Environ. Saf., 2017, 143, 210–216 CrossRef CAS PubMed.
- L. A. Henriquez-Hernandez, D. Montero, M. Camacho, R. Gines, L. D. Boada, B. R. Bordon, P. F. Valeron, M. Almeida-Gonzalez, M. Zumbado, R. Haroun and O. P. Luzardo, Comparative analysis of selected semi-persistent and emerging pollutants in wild-caught fish and aquaculture associated fish using Bogue (Boops boops) as sentinel species, Sci. Total Environ., 2017, 581, 199–208 CrossRef PubMed.
- X. Z. Peng, Y. J. Fan, J. B. Jin, S. S. Xiong, J. Liu and C. M. Tang, Bioaccumulation and biomagnification of ultraviolet absorbents in marine wildlife of the Pearl River Estuarine, South China Sea, Environ. Pollut., 2017, 225, 55–65 CrossRef CAS PubMed.
- F. R. Chen, C. Huber and P. Schroder, Fate of the sunscreen compound oxybenzone in Cyperus alternifolius based hydroponic culture: Uptake, biotransformation and phytotoxicity, Chemosphere, 2017, 182, 638–646 CrossRef CAS PubMed.
- European Chemicals Agency, Information on Chemicals, https://echa.europa.eu/information-on-chemicals/evaluation/community-rolling-action-plan/corap-table/, updated January 15, 2018, accessed September 14, 2017.
- K. P. Lawrence, P. F. Long and A. R. Young, Mycosporine-like amino acids for skin photoprotection, Curr. Med. Chem., 2017, 24, 1–16 CrossRef PubMed.
- R. Lindsay and A. Schweiger, Arctic sea ice thickness loss determined using subsurface, aircraft, and satellite observations, Cryosphere, 2015, 9, 269–283 CrossRef.
- M. C. Serreze and J. Stroeve, Arctic sea ice trends, variability and implications for seasonal ice forecasting, Philos. Trans. R. Soc., A, 2015, 373(2045), 20140159 CrossRef PubMed.
- E. Schuur, A. McGuire, C. Schädel, G. Grosse, J. Harden, D. Hayes, G. Hugelius, C. Koven, P. Kuhry and D. Lawrence, Climate change and the permafrost carbon feedback, Nature, 2015, 520, 171–179 CrossRef CAS PubMed.
- C. J. Cox, R. S. Stone, D. C. Douglas, D. M. Stanitski, G. J. Divoky, G. S. Dutton, C. Sweeney, J. Craig George and D. U. Longenecker, Drivers and environmental responses to the changing annual snow cycle of northern Alaska, Bull. Am. Meteorol. Soc., 2018 DOI:10.1175/BAMS-D-16-0201.1.
- T. Šmejkalová, M. E. Edwards and J. Dash, Arctic lakes show strong decadal trend in earlier spring ice-out, Sci. Rep., 2016, 6, 38449 CrossRef PubMed.
- C. P. Ward and R. M. Cory, Complete and partial photo-oxidation of dissolved organic matter draining permafrost soils, Environ. Sci. Technol., 2016, 50, 3545–3553 CrossRef CAS PubMed.
- S. M. Stackpoole, D. E. Butman, D. W. Clow, K. L. Verdin, B. V. Gaglioti, H. Genet and R. G. Striegl, Inland waters and their role in the carbon cycle of Alaska, Ecol. Appl., 2017, 27, 1403–1420 CrossRef PubMed.
- D. Vachon, J. F. Lapierre and P. A. Giorgio, Seasonality of photochemical dissolved organic carbon mineralization and its relative contribution to pelagic CO2 production in northern lakes, J. Geophys. Res.: Biogeosci., 2016, 121, 864–878 CrossRef CAS.
- R. M. Cory, C. P. Ward, B. C. Crump and G. W. Kling, Sunlight controls water column processing of carbon in arctic fresh waters, Science, 2014, 345, 925–928 CrossRef CAS PubMed.
- A. Stubbins, P. J. Mann, L. Powers, T. B. Bittar, T. Dittmar, C. P. McIntyre, T. I. Eglinton, N. Zimov and R. G. Spencer, Low photolability of yedoma permafrost dissolved organic carbon, J. Geophys. Res.: Biogeosci., 2017, 122, 200–211 CrossRef CAS.
- C. P. Ward, S. G. Nalven, B. C. Crump, G. W. Kling and R. M. Cory, Photochemical alteration of organic carbon draining permafrost soils shifts microbial metabolic pathways and stimulates respiration, Nat. Commun., 2017, 8, 772 CrossRef PubMed.
- V. J. Hill and R. C. Zimmerman, Characteristics of colored dissolved organic material in first year landfast sea ice and the underlying water column in the Canadian Arctic in the early spring, Mar. Chem., 2016, 180, 1–13 CrossRef CAS.
- A. D. McGuire, L. G. Anderson, T. R. Christensen, S. Dallimore, L. Guo, D. J. Hayes, M. Heimann, T. D. Lorenson, R. W. Macdonald and N. Roulet, Sensitivity of the carbon cycle in the Arctic to climate change, Ecol. Monogr., 2009, 79, 523–555 CrossRef.
- R. G. M. Spencer, P. J. Mann, T. Dittmar, T. I. Eglinton, C. McIntyre, R. M. Holmes, N. Zimov and A. Stubbins, Detecting the signature of permafrost thaw in Arctic rivers, Geophys. Res. Lett., 2015, 42, 2830–2835 CrossRef.
- D. C. Waggoner, A. S. Wozniak, R. M. Cory and P. G. Hatcher, The role of reactive oxygen species in the degradation of lignin derived dissolved organic matter, Geochim. Cosmochim. Acta, 2017, 208, 171–184 CrossRef CAS.
- D. H. Bromwich, J. P. Nicolas, A. J. Monaghan, M. A. Lazzara, L. M. Keller, G. A. Weidner and A. B. Wilson, Central West Antarctica among the most rapidly warming regions on Earth, Nat. Geosci., 2013, 6, 139–145 CrossRef CAS.
- J. R. Lee, B. Raymond, T. J. Bracegirdle, I. Chadès, R. A. Fuller, J. D. Shaw and A. Terauds, Climate change drives expansion of Antarctic ice-free habitat, Nature, 2017, 547, 49–54 CrossRef CAS PubMed.
- M. N. Gooseff, J. E. Barrett, B. J. Adams, P. T. Doran, A. G. Fountain, W. B. Lyons, D. M. McKnight, J. C. Priscu, E. R. Sokol, C. Takacs-Vesbach, M. L. Vandegehuchte, R. A. Virginia and D. H. Wall, Decadal ecosystem response to an anomalous melt season in a polar desert in Antarctica, Nat. Ecol. Evolut., 2017, 1, 1334–1338 CrossRef PubMed.
- R. Antony, A. S. Willoughby, A. M. Grannas, V. Catanzano, R. L. Sleighter, M. Thamban, P. G. Hatcher and S. Nair, Molecular insights on dissolved organic matter transformation by supraglacial microbial communities, Environ. Sci. Technol., 2017, 51, 4328–4337 CrossRef CAS PubMed.
- A. Ahlström, M. R. Raupach, G. Schurgers, B. Smith, A. Arneth, M. Jung, M. Reichstein, J. G. Canadell, P. Friedlingstein and A. K. Jain, The dominant role of semi-arid ecosystems in the trend and variability of the land CO2 sink, Science, 2015, 348, 895–899 CrossRef PubMed.
- A. Gaxiola and J. J. Armesto, Understanding litter decomposition in semiarid ecosystems: linking leaf traits, UV exposure and rainfall variability, Front. Plant. Sci., 2015, 6, 140 Search PubMed.
- N. R. Baker and S. D. Allison, Ultraviolet photodegradation facilitates microbial litter decomposition in a Mediterranean climate, Ecol., 2015, 96, 1994–2003 Search PubMed.
- G. Huang, H. M. Zhao and Y. Li, Litter decomposition in hyper-arid deserts: Photodegradation is still important, Sci. Total Environ., 2017, 601–602, 784–792 CrossRef CAS PubMed.
- D. Gliksman, A. Rey, R. Seligmann, R. Dumbur, O. Sperling, Y. Navon, S. Haenel, P. De Angelis, J. A. Arnone III and J. M. Grünzweig, Biotic degradation at night, abiotic degradation at day: positive feedbacks on litter decomposition in drylands, Glob. Change Biol., 2017, 23, 1564–1574 CrossRef PubMed.
- A. Demidov, S. Sheberstov, V. Gagarin and P. Khlebopashev, Seasonal variation of the satellite-derived phytoplankton primary production in the Kara Sea, Oceanology, 2017, 57, 91–104 CrossRef.
- C. Romera-Castillo, R. T. Letscher and D. A. Hansell, New nutrients exert fundamental control on dissolved organic carbon accumulation in the surface Atlantic Ocean, Proc. Natl. Acad. Sci. U. S. A, 2016, 113, 10497–10502 CrossRef CAS PubMed.
- W. Fu, J. T. Randerson and J. K. Moore, Climate change impacts on net primary production (NPP) and export production (EP) regulated by increasing stratification and phytoplankton community structure in the CMIP5 models, Biogeosciences, 2016, 13, 5151 Search PubMed.
- D. Richardson, S. Melles, R. Pilla, A. Hetherington, L. Knoll, C. Williamson, B. Kraemer, J. Jackson, E. Long, K. Moore, L. Rudstam, J. Rusak, J. Saros, S. Sharma, K. Strock, K. Weathers and C. Wigdahl-Perry, Transparency, geomorphology and mixing regime explain variability in trends in lake temperature and stratification across Northeastern North America (1975–2014), Water, 2017, 9, 442 CrossRef.
- R. T. Letscher and J. K. Moore, Modest net autotrophy in the oligotrophic ocean, Glob. Biogeochem. Cycles, 2017, 31, 699–708 CrossRef CAS.
- S. J. Traving, O. Rowe, N. M. Jakobsen, H. Sørensen, J. Dinasquet, C. A. Stedmon, A. Andersson and L. Riemann, The effect of increased loads of dissolved organic matter on estuarine microbial community composition and function, Front. Microbiol., 2017, 8, 00351 Search PubMed.
- J. E. Vonk, S. E. Tank, W. B. Bowden, I. Laurion, W. F. Vincent, P. Alekseychik, M. Amyot, M. Billet, J. Canario and R. M. Cory, Reviews and syntheses: Effects of permafrost thaw on Arctic aquatic ecosystems, Biogeosciences, 2015, 12, 7129–7167 CrossRef.
- J. E. Overland, K. Dethloff, J. A. Francis, R. J. Hall, E. Hanna, S.-J. Kim, J. A. Screen, T. G. Shepherd and T. Vihma, Nonlinear response of mid-latitude weather to the changing Arctic, Nat. Clim. Change, 2016, 6, 992–999 CrossRef.
- N. J. Byrne, T. G. Shepherd, T. Woollings and R. A. Plumb, Nonstationarity in Southern Hemisphere climate variability associated with the seasonal breakdown of the stratospheric polar vortex, J. Clim., 2017, 30, 7125–7139 CrossRef.
- C. E. H. Kissman, C. E. Williamson, K. C. Rose and J. E. Saros, Nutrients associated with terrestrial dissolved organic matter drive changes in zooplankton:phytoplankton biomass ratios in an alpine lake, Freshwater Biol., 2017, 62, 40–51 CrossRef CAS.
- D. A. Hutchins and P. W. Boyd, Marine phytoplankton and the changing ocean iron cycle, Nat. Clim. Change, 2016, 6, 1072–1079 CrossRef CAS.
- D. Shi, S. A. Kranz, J.-M. Kim and F. M. M. Morel, Ocean acidification slows nitrogen fixation and growth in the dominant diazotroph Trichodesmium under low-iron conditions, Proc. Natl. Acad. Sci. U. S. A, 2012, 109, E3094–E3100 CrossRef CAS PubMed.
- B. Sulzberger, Light-Induced redox cycling of iron: Roles for CO2 uptake and release by aquatic ecosystems, Aquat. Geochem., 2015, 21, 65–80 CrossRef CAS.
- C. Voigt, R. E. Lamprecht, M. E. Marushchak, S. E. Lind, A. Novakovskiy, M. Aurela, P. J. Martikainen and C. Biasi, Warming of subarctic tundra increases emissions of all three important greenhouse gases–carbon dioxide, methane, and nitrous oxide, Glob. Change Biol., 2017, 23, 3121–3138 CrossRef PubMed.
- D. R. N. Brown, M. T. Jorgenson, K. Kielland, D. L. Verbyla, A. Prakash and J. C. Koch, Landscape effects of wildfire on permafrost distribution in interior Alaska derived from remote sensing, Rem.e Sens., 2016, 8, 1–22 Search PubMed.
- B. Kim and S. Sarkar, Impact of wildfires on some greenhouse gases over continental USA: A study based on satellite data, Rem. Sens. Environ., 2017, 188, 118–126 CrossRef.
- K. Mopper, D. J. Kieber and A. Stubbins, Marine Photochemistry of Organic Matter: Processes and Impacts, in Biogeochemistry of Dissolved Organic Matter ed. D. A. Hansell and C. A. Carlson, Elsevier, Amsterdam, 2nd edn, 2015, pp. 389–450 Search PubMed.
- C. Chu, R. A. Lundeen, C. K. Remucal, M. Sander and K. McNeill, Enhanced indirect photochemical transformation of histidine and histamine through association with chromophoric dissolved organic matter, Environ. Sci. Technol., 2015, 49, 5511–5519 CrossRef CAS PubMed.
- A. R. A. Soares, A. K. Bergström, R. A. Sponseller, J. M. Moberg, R. Giesler, E. S. Kritzberg, M. Jansson and M. Berggren, New insights on resource stoichiometry: assessing availability of carbon, nitrogen, and phosphorus to bacterioplankton, Biogeosciences, 2017, 14, 1527–1539 Search PubMed.
- F. Paulot, D. J. Jacob, M. T. Johnson, T. G. Bell, A. R. Baker, W. C. Keene, I. D. Lima, S. C. Doney and C. A. Stock, Global oceanic emission of ammonia: Constraints from seawater and atmospheric observations, Global Biogeochem. Cycles, 2015, 29, 1165–1178 CrossRef CAS.
- J. Wang, L. Liu, X. Wang and Y. Chen, The interaction between abiotic photodegradation and microbial decomposition under ultraviolet radiation, Global Change Biol., 2015, 21, 2095–2104 CrossRef PubMed.
- M. S. Khosh, J. W. McClelland, A. D. Jacobson, T. A. Douglas, A. J. Barker and G. O. Lehn, Seasonality of dissolved nitrogen from spring melt to fall freezeup in Alaskan Arctic tundra and mountain streams, J. Geophys. Res.: Biogeosci., 2017, 122, 1718–1737 CrossRef CAS.
- L. C. Bodhipaksha, C. M. Sharpless, Y. P. Chin and A. A. MacKay, Role of effluent organicmatter in the photochemical degradation of compounds of wastewater origin, Water Res., 2017, 110, 170–179 CrossRef CAS PubMed.
- M. C. Semones, C. M. Sharpless, A. A. MacKay and Y. P. Chin, Photodegradation of UV filters oxybenzone and sulisobenzone in wastewater effluent and by dissolved organic matter, Appl. Geochem., 2017, 83, 150–157 CrossRef CAS.
- H. Dong, Z. Qiang, J. Lian and J. Qu, Degradation of nitro-based pharmaceuticals by UV photolysis: Kinetics and simultaneous reduction on halonitromethanes formation potential, Water Res., 2017, 119, 83–90 CrossRef CAS PubMed.
- R. A. Lundeen, C. Chu, M. Sander and K. McNeill, Photooxidation of the antimicrobial, nonribosomal peptide Bacitracin A by singlet oxygen under environmentally relevant conditions, Environ. Sci. Technol., 2016, 50, 8586–8595 CrossRef CAS PubMed.
- Z. Qiao and K. R. Wigginton, Direct and indirect photochemical reactions in viral RNA measured with RT-qPCR and mass spectrometry, Environ. Sci. Technol., 2016, 50, 13371–13379 CrossRef CAS PubMed.
- C. Y. Chen and R. G. Zepp, Probing photosensitization by functionalized carbon nanotubes, Environ. Sci. Technol., 2015, 49, 13835–13843 CrossRef CAS PubMed.
- W. C. Hou, W. M. Henderson, I. Chowdhury, D. G. Goodwin, X. J. Chang, S. Martin, D. H. Fairbrother, D. Bouchard and R. G. Zepp, The contribution of indirect photolysis to the degradation of graphene oxide in sunlight, Carbon, 2016, 110, 426–437 CrossRef CAS.
- T. Nguyen, E. J. Petersen, B. Pellegrin, J. M. Gorham, T. Lam, M. Zhao and L. Sung, Impact of UV irradiation on multiwall carbon nanotubes in nanocomposites: Formation of entangled surface layer and mechanisms of release resistance, Carbon, 2017, 116, 191–200 CrossRef CAS PubMed.
- W. Wohlleben, C. Kingston, J. Carter, E. Sahle-Demessie, S. Vázquez-Campos, B. Acrey, C.-Y. Chen, E. Walton, H. Egenolf, P. Müller and R. G. Zepp, NanoRelease: Pilot interlaboratory comparison of a weathering protocol applied to resilient and labile polymers with and without embedded carbon nanotubes, Carbon, 2017, 113, 346–360 CrossRef CAS.
- L. Carena, M. Minella, F. Barsotti, M. Brigante, M. Milan, A. Ferrero, S. Berto, C. Minero and D. Vione, Phototransformation of the herbicide propanil in paddy field water, Environ. Sci. Technol., 2017, 51, 2695–2704 CrossRef CAS PubMed.
- M. McConville, N. M. Cohen, S. M. Nowicki, S. R. Lantz, J. L. Hixson, A. S. Ward and C. K. Remucal, A field analysis of lampricide photodegradation in Great Lakes tributaries, Environ. Sci.: Processes Impacts, 2017, 19, 891–900 CAS.
- M. B. McConville, S. P. Mezyk and C. K. Remucal, Indirect photodegradation of the lampricides TFM and niclosamide, Environ. Sci.: Processes Impacts, 2017, 19, 1028–1039 CAS.
- M. Bodrato and D. Vione, APEX (Aqueous Photochemistry of Environmentally occurring Xenobiotics): a free software tool to predict the kinetics of photochemical processes in surface waters, Environ. Sci.: Processes Impacts, 2014, 16, 732–740 CAS.
- T. Kohn, M. J. Mattle, M. Minella and D. Vione, A modeling approach to estimate the solar disinfection of viral indicator organisms in waste stabilization ponds and surface waters, Water Res., 2016, 88, 912–922 CrossRef CAS PubMed.
- M. J. Mattle, D. Vione and T. Kohn, Conceptual model and experimental framework to determine the contributions of direct and indirect photoreactions to the solar disinfection of MS2, phiX174, and adenovirus, Environ. Sci. Technol., 2015, 49, 334–342 CrossRef CAS PubMed.
- P. J. Young, A. T. Archibald, K. W. Bowman, J. F. Lamarque, V. Naik, D. S. Stevenson, S. Tilmes, A. Voulgarakis, O. Wild, D. Bergmann, P. Cameron-Smith, I. Cionni, W. J. Collins, S. B. Dalsøren, R. M. Doherty, V. Eyring, G. Faluvegi, L. W. Horowitz, B. Josse, Y. H. Lee, I. A. MacKenzie, T. Nagashima, D. A. Plummer, M. Righi, S. T. Rumbold, R. B. Skeie, D. T. Shindell, S. A. Strode, K. Sudo, S. Szopa and G. Zeng, Pre-industrial to end 21st century projections of tropospheric ozone from the Atmospheric Chemistry and Climate Model Intercomparison Project (ACCMIP), Atmos. Chem. Phys., 2013, 13, 2063–2090 Search PubMed.
- A. C. Boothe and C. R. Homeyer, Global large-scale stratosphere–troposphere exchange in modern reanalyses, Atmos. Chem. Phys., 2017, 17, 5537–5559 CrossRef CAS.
- J. W. Greenslade, S. P. Alexander, R. Schofield, J. A. Fisher and A. K. Klekociuk, Stratospheric ozone intrusion events and their impacts on tropospheric ozone in the Southern Hemisphere, Atmos. Chem. Phys., 2017, 17, 10269–10290 CAS.
- T. P. Canty, L. Hembeck, T. P. Vinciguerra, D. C. Anderson, D. L. Goldberg, S. F. Carpenter, D. J. Allen, C. P. Loughner, R. J. Salawitch and R. R. Dickerson, Ozone and NOx chemistry in the eastern US: evaluation of CMAQ/CB05 with satellite (OMI) data, Atmos. Chem. Phys., 2015, 15, 10965–10982 Search PubMed.
- K. R. Travis, D. J. Jacob, J. A. Fisher, P. S. Kim, E. A. Marais, L. Zhu, K. Yu, C. C. Miller, R. M. Yantosca, M. P. Sulprizio, A. M. Thompson, P. O. Wennberg, J. D. Crounse, J. M. St Clair, R. C. Cohen, J. L. Laughner, J. E. Dibb, S. R. Hall, K. Ullmann, G. M. Wolfe, I. B. Pollack, J. Peischl, J. A. Neuman and X. Zhou, Why do models overestimate surface ozone in the Southeast United States?, Atmos. Chem. Phys., 2016, 16, 13561–13577 CAS.
- Y. Zhang, O. R. Cooper, A. Gaudel, A. M. Thompson, P. Nedelec, S.-Y. Ogino and J. J. West, Tropospheric ozone change from 1980 to 2010 dominated by equatorward redistribution of emissions, Nat. Geosci., 2016, 9, 875–879 CrossRef CAS.
- E. Dlugokencky, Trends in atmospheric methane, NOAA/ESRL, http://www.esrl.noaa.gov/gmd/ccgg/trends_ch4/, accessed 14 Sept 2017.
- J. McNorton, M. P. Chipperfield, M. Gloor, C. Wilson, W. Feng, G. D. Hayman, M. Rigby, P. B. Krummel, S. Doherty, R. G. Prinn, R. F. Weiss, D. Young, E. Dlugokencky and S. A. Montzka, Role of OH variability in the stalling of the global atmospheric CH4 growth rate from 1999 to 2006, Atmos. Chem. Phys., 2016, 16, 7943–7956 CAS.
- M. Rigby, S. A. Montzka, R. G. Prinn, J. W. C. White, D. Young, S. O'Doherty, M. F. Lunt, A. L. Ganesan, A. J. Manning, P. G. Simmonds, P. K. Salameh, C. M. Harth, J. Muhle, R. F. Weiss, P. J. Fraser, L. P. Steele, P. B. Krummel, A. McCulloch and S. Park, Role of atmospheric oxidation in recent methane growth, Proc. Natl. Acad. Sci. U. S. A., 2017, 114, 5373–5377 CrossRef CAS PubMed.
- M. C. Turner, D. Krewski, W. R. Diver, C. A. PopeIII, R. T. Burnett, M. Jerrett, J. D. Marshall and S. M. Gapstur, Ambient air pollution and cancer mortality in the Cancer Prevention Study II, Environ. Health Perspect., 2017, 125, 087013 CrossRef PubMed.
- J. Pollmann, D. Helmig, D. Liptzin, C. R. Thompson, J. Hueber, P. P. Tans and J. Lelieveld, Variability analyses, site characterization, and regional [OH] estimates using trace gas measurements from the NOAA Global Greenhouse Gas Reference Network, Elementa Sci. Anthropoc., 2016, 4, 000128 Search PubMed.
- S. A. Strode, H. M. Worden, M. Damon, A. R. Douglass, B. N. Duncan, L. K. Emmons, J.-F. Lamarque, M. Manyin, L. D. Oman, J. M. Rodriguez, S. E. Strahan and S. Tilmes, Interpreting space-based trends in carbon monoxide with multiple models, Atmos. Chem. Phys., 2016, 16, 7285–7294 CAS.
- M. J. Newland, P. Martinerie, E. Witrant, D. Helmig, D. R. Worton, C. Hogan, W. T. Sturges and C. E. Reeves, Changes to the chemical state of the Northern Hemisphere atmosphere during the second half of the twentieth century, Atmos. Chem. Phys., 2017, 17, 8269–8283 CAS.
- K. Adachi, S. H. Chung and P. R. Buseck, Shapes of soot aerosol particles and implications for their effects on climate, J. Geophys. Res., 2010, 115, D15206 CrossRef.
- O. M. Morakinyo, M. I. Mokgobu, M. S. Mukhola and R. P. Hunter, Health outcomes of exposure to biological and chemical components of inhalable and respirable particulate matter, Int. J. Environ. Res. Public Health, 2016, 13, 592 CrossRef PubMed.
- K. Ueda, M. Yamagami, F. Ikemori, K. Hisatsune and H. Nitta, Associations between fine particulate matter components and daily mortality in Nagoya, Japan, J. Epidemiol., 2016, 26, 249–257 CrossRef PubMed.
- IARC, Outdoor air pollution, IARC Monogr Eval Carcinog Risk Hum, 2016, 109, 1–454 Search PubMed.
- A. J. Cohen, M. Brauer, R. Burnett, H. R. Anderson, J. Frostad, K. Estep, K. Balakrishnan, B. Brunekreef, L. Dandona, R. Dandona, V. Feigin, G. Freedman, B. Hubbell, A. Jobling, H. Kan, L. Knibbs, Y. Liu, R. Martin, L. Morawska, C. A. Pope 3rd, H. Shin, K. Straif, G. Shaddick, M. Thomas, R. van Dingenen, A. van Donkelaar, T. Vos, C. J. L. Murray and M. H. Forouzanfar, Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: An analysis of data from the Global Burden of Diseases Study 2015, Lancet, 2017, 389, 1907–1918 CrossRef.
- W. Miao, X. Huang and Y. Song, An economic assessment of the health effects and crop yield losses caused by air pollution in mainland China, J. Environ. Sci., 2017, 56, 102–113 CrossRef PubMed.
- S. D. Ghude, C. K. Jena, R. Kumar, S. Kulkarni and D. Chate, Impact of emission mitigation on ozone-induced wheat and rice damage in India, Curr. Sci., 2016, 110, 1452–1458 CAS.
- S. Lal, S. Venkataramani, M. Naja, J. C. Kuniyal, T. K. Mandal, P. K. Bhuyan, K. M. Kumari, S. N. Tripathi, U. Sarkar, T. Das, Y. V. Swamy, K. R. Gopal, H. Gadhavi and M. K. S. Kumar, Loss of crop yields in India due to surface ozone: An estimation based on a network of observations, Environ. Sci. Pollut. Res., 2017, 24, 20972–20981 CrossRef CAS PubMed.
- V. Suganthy and C. Udayasoorian, Assessing the impact of ambient ozone (O3) on the growth and yield of potato genotypes (Solanum tuberosum L.) by using exposure indices over the high altitude of western Ghats location in Southern India, Asian J. Environ. Sci., 2016, 11, 102–105 CrossRef.
- P. E. Karlsson, J. Klingberg, M. Engardt, C. Andersson, J. Langner, G. P. Karlsson and H. Pleijel, Past, present and future concentrations of ground-level ozone and potential impacts on ecosystems and human health in northern Europe, Sci.Total Environ., 2017, 576, 22–35 CrossRef CAS PubMed.
- K. Solomon, G. Velders, S. Wilson, S. Madronich, J. Longstreth, P. Aucamp and J. Bornman, Sources, fates, toxicity, and risks of trifluoroacetic acid and its salts: Relevance to substances regulated under the Montreal and Kyoto protocols, J. Toxicol. Environ. Health, Part B, 2016, 19, 289–304 CAS.
- R. Qu, J. Liu, C. Li, L. Wang, Z. Wang and J. Wu, Experimental and theoretical insights into the photochemical decomposition of environmentally persistent perfluorocarboxylic acids, Water Res., 2016, 104, 34–43 CrossRef CAS PubMed.
- J. Guo, Z. Zhai, L. Wang, Z. Wang, J. Wu, B. Zhang and J. Zhang, Dynamic and thermodynamic mechanisms of TFA adsorption by particulate matter, Environ. Pollut., 2017, 225, 175–183 CrossRef CAS PubMed.
- M. Scheurer, K. Nödler, F. Freeling, J. Janda, O. Happel, M. Riegel, U. Müller, F. R. Storck, M. Fleig, F. T. Lange, A. Brunsch and H.-J. Brauch, Small, mobile, persistent: Trifluoroacetate in the water cycle – overlooked sources, pathways, and consequences for drinking water supply, Water Res., 2017, 126, 460–471 CrossRef CAS PubMed.
- European Community, Council Directive 98/83/EC of 3 November 1998 on the quality of water intended for human consumption, Off. J. Eur. Communities, 1998, L 330, 32–54 Search PubMed.
- T.-C. Chang and S.-T. Chang, Multiple photostabilization actions of heartwood extract from Acacia confusa, Wood Sci. Technol., 2017, 51, 1133–1153 CrossRef CAS.
- W. J. Grigsby, Simulating the protective role of bark proanthocyanidins in surface coatings: Unexpected beneficial photo-stabilisation of exposed timber surfaces, Prog. Org. Coat., 2017, 110, 55–61 CrossRef CAS.
- W. Grigsby and D. Steward, Applying the protective role of condensed tannins to acrylic-based surface coatings exposed to accelerated weathering, J. Polym. Environ., 2017, 1–11 Search PubMed.
- D. Ye, S. Li, X. Lu, X. Zhang and O. J. Rojas, Antioxidant and thermal stabilization of polypropylene by addition of butylated lignin at low loadings, ACS Sustainable Chem. Eng., 2016, 4, 5248–5257 CrossRef CAS.
- D. Liu, Y. Li, Y. Qian, Y. Xiao, S. Du and X. Qiu, Synergistic antioxidant performance of lignin and quercetin mixtures, ACS Sustainable Chem. Eng., 2017, 5, 8424–8428 CrossRef CAS.
- F. Chen, W. Liu, S. I. Seyed Shahabadi, J. Xu and X. Lu, Sheet-like lignin particles as multifunctional fillers in polypropylene, ACS Sustainable Chem. Eng., 2016, 4, 4997–5004 CrossRef CAS.
- R. Gadioli, W. R. Waldman and M. A. De Paoli, Lignin as a green primary antioxidant for polypropylene, J. Appl. Polym. Sci., 2016, 133, 43558 CrossRef.
- A. S. Kabir, Effects of lignin as a stabilizer or antioxidant in polyolefins, Masters thesis, The University of Western Ontario, London, ON, Canada, 2017 Search PubMed.
- G. Capobianco, M. P. Bracciale, D. Sali, F. Sbardella, P. Belloni, G. Bonifazi, S. Serranti, M. L. Santarelli and M. C. Guidi, Chemometrics approach to FT-IR hyperspectral imaging analysis of degradation products in artwork cross-section, Microchem. J., 2017, 132, 69–76 CrossRef CAS.
- H. Turgut-Sahin and G. I. Mantanis, Colour changes of pine and fir wood treated with several titanium and zinc-oxide based nanocompounds, Adv. Forestry Lett., 2016, 5, 17–23 CrossRef.
- K. Srinivas and K. K. Pandey, Enhancing photostability of wood coatings using titanium dioxide nanoparticles, in Wood is Good, Springer, 2017, pp. 251–259 Search PubMed.
- R. Moya, A. Rodríguez-Zúñiga, J. Vega-Baudrit and A. Puente-Urbina, Effects of adding TiO2 nanoparticles to a water-based varnish for wood applied to nine tropical woods of Costa Rica exposed to natural and accelerated weathering, J. Coat. Technol. Res., 2017, 14, 141–152 CrossRef CAS.
- L. Kong, K. Tu, H. Guan and X. Wang, Growth of high-density ZnO nanorods on wood with enhanced photostability, flame retardancy and water repellency, Appl. Surf. Sci., 2017, 407, 479–484 CrossRef CAS.
- D. Varga, L. Tolvaj, S. Tsuchikawa, L. Bejo and E. Preklet, Temperature dependence of wood photodegradation monitored by infrared spectroscopy, J. Photochem. Photobiol., A, 2017, 348, 219–225 CrossRef CAS.
- K. A. Smeland, K. H. Liland, J. Sandak, A. Sandak, L. R. Gobakken, T. K. Thiis and I. Burud, Near infrared hyperspectral imaging in transmission mode: assessing the weathering of thin wood samples, J. Near Infrared Spectrosc., 2016, 24, 595–604 CrossRef CAS.
- I. Rafique, A. Kausar and B. Muhammad, Epoxy resin composite reinforced with carbon fiber and inorganic filler: Overview on preparation and properties, Polym.-Plast. Technol. Eng., 2016, 55, 1653–1672 CrossRef CAS.
- P. Fei, H. Xiong, J. Cai, C. Liu and Y. Yu, Enhanced the weatherability of bamboo fiber-based outdoor building decoration materials by rutile nano-TiO2, Constr. Build. Mater., 2016, 114, 307–316 CrossRef CAS.
- Y. Peng, W. Wang and J. Cao, Preparation of lignin–clay complexes and its effects on properties and weatherability of wood flour/polypropylene composites, Ind. Eng. Chem. Res., 2016, 55, 9657–9666 CrossRef CAS.
- H. Ben Hadj Salah, H. Ben Daly, J. Denault and F. Perrin, UV degradation of clay-reinforced polypropylene nanocomposites, Polym. Eng. Sci., 2016, 56, 469–478 CAS.
- C. Kaynak and B. Sarı, Accelerated weathering performance of polylactide and its montmorillonite nanocomposite, Appl. Clay Sci., 2016, 121, 86–94 CrossRef.
- H. Xiu, X. Qi, H. Bai, Q. Zhang and Q. Fu, Simultaneously improving toughness and UV-resistance of polylactide/titanium dioxide nanocomposites by adding poly (ether) urethane, Polym. Degrad. Stab., 2017, 143, 136–144 CrossRef CAS.
- T. V. Nguyen, P. N. Tri, T. D. Nguyen, R. El Aidani, V. T. Trinh and C. Decker, Accelerated degradation of water borne acrylic nanocomposites used in outdoor protective coatings, Polym. Degrad. Stab., 2016, 128, 65–76 CrossRef CAS.
- G. Di Pasquale and A. Pollicino, Properties of polystyrene clay nanocomposites prepared using two new imidazolium surfactants, J. Nanomater., 2017 DOI:10.1155/2017/2594958.
- P. A. Zapata, A. Zenteno, N. Amigó, F. M. Rabagliati, F. Sepúlveda, F. Catalina and T. Corrales, Study on the photodegradation of nanocomposites based on polypropylene and TiO2 nanotubes, Polym. Degrad. Stab., 2016, 133, 101–107 CrossRef CAS.
- S. Harper, W. Wohlleben, M. Doa, B. Nowack, S. Clancy, R. Canady and A. Maynard, Measuring nanomaterial release from carbon nanotube composites: Review of the state of the science, J. Phys.: Conf. Ser., 2015, 617, 012026 CrossRef.
- M. Kovochich, C.-C. D. Fung, R. Avanasi and A. K. Madl, Review of techniques and studies characterizing the release of carbon nanotubes from nanocomposites: Implications for exposure and human health risk assessment, J. Exposure Sci. Environ. Epidemiol., 2017 DOI:10.1038/jes.2017.6.
- J. Li, Multiwalled carbon nanotubes reinforced polypropylene composite material, J. Nanomater., 2017, 2017 Search PubMed.
- M. H. Al-Saleh, Carbon nanotube-filled polypropylene/polyethylene blends: Compatibilization and electrical properties, Polym. Bull., 2016, 73, 975–987 CrossRef CAS.
- R. S. Lankone, J. Wang, J. F. Ranville and D. H. Fairbrother, Photodegradation of polymer-CNT nanocomposites: effect of CNT loading and CNT release characteristics, Environ. Sci.: Nano, 2017, 4, 967–982 RSC.
- S.-W. Lee, S. Kim, S. Bae, K. Cho, T. Chung, L. E. Mundt, S. Lee, S. Park, H. Park and M. C. Schubert, UV degradation and recovery of perovskite solar cells, Sci. Rep., 2016, 6, 38150 CrossRef CAS PubMed.
- E. Polydorou, I. Sakellis, A. Soultati, A. Kaltzoglou, T. A. Papadopoulos, J. Briscoe, D. Tsikritzis, M. Fakis, L. C. Palilis and S. Kennou, Avoiding ambient air and light induced degradation in high-efficiency polymer solar cells by the use of hydrogen-doped zinc oxide as electron extraction material, Nano Energy, 2017, 34, 500–514 CrossRef CAS.
- A. Badiee, I. Ashcroft and R. D. Wildman, The thermo-mechanical degradation of ethylene vinyl acetate used as a solar panel adhesive and encapsulant, Int. J. Adhes. Adhes., 2016, 68, 212–218 CrossRef CAS.
- M. H. Kim, H. S. Eom, D.-J. Byun and K.-Y. Choi, Photodegradation behavior of ethylene/vinyl acetate copolymer (EVA) film for solar cell encapsulant, Polymer, 2016, 40, 477–482 CAS.
- C.-C. Lin, P. J. Krommenhoek, S. S. Watson and X. Gu, Depth profiling of degradation of multilayer photovoltaic backsheets after accelerated laboratory weathering: Cross-sectional Raman imaging, Sol. Energy Mater. Sol. Cells, 2016, 144, 289–299 CrossRef CAS.
- M. Malaki, Y. Hashemzadeh and M. Karevan, Effect of nano-silica on the mechanical properties of acrylic polyurethane coatings, Prog. Org. Coat., 2016, 101, 477–485 CrossRef CAS.
- B. Ottersböck, G. Oreski and G. Pinter, Comparison of different microclimate effects on the aging behavior of encapsulation materials used in photovoltaic modules, Polym. Degrad. Stab., 2017, 138, 182–191 CrossRef.
- M. Bag, S. Banerjee, R. Faust and D. Venkataraman, Self-healing polymer sealant for encapsulating flexible solar cells, Sol. Energy Mater. Sol. Cells, 2016, 145, 418–422 CrossRef CAS.
- I. Topolniak, A. Chapel, J. Gaume, P.-O. Bussiere, G. Chadeyron, J.-L. Gardette and S. Therias, Applications of polymer nanocomposites as encapsulants for solar cells and LEDs: Impact of photodegradation on barrier and optical properties, Polym. Degrad. Stab., 2017, 145, 52–59 CrossRef CAS.
- A. L. Andrady, The plastic in microplastics: A review, Mar. Pollut. Bull., 2017, 119, 12–22 CrossRef CAS PubMed.
- J. Gigault, B. Pedrono, B. Maxit and A. Ter Halle, Marine plastic litter: The unanalyzed nano-fraction, Environ. Sci.: Nano, 2016, 3, 346–350 RSC.
- T. Galloway and C. Lewis, Marine microplastics, Curr. Biol., 2017, 27, R445–R446 CrossRef CAS PubMed.
- S. Lambert and M. Wagner, Formation of microscopic particles during the degradation of different polymers, Chemosphere, 2016, 161, 510–517 CrossRef CAS PubMed.
- N. Kalogerakis, K. Karkanorachaki, G. Kalogerakis, E. Triantafyllidi, A. Gotsis, P. Partsinevelos and F. Fava, Microplastics generation: Onset of fragmentation of polyethylene films in marine environment mesocosms, Front. Mar. Sci., 2017, 4, 00084 Search PubMed.
- M. Zhang, B. Tang, L. Sun and X. Wang, Protection of silica-coated ZnO nanoparticles on pre-dyed polyester fabrics against photofading, J. Text. Inst., 2017, 108, 95–101 CrossRef CAS.
- D. Gao, L. Lyu, B. Lyu, J. Ma, L. Yang and J. Zhang, Multifunctional cotton fabric loaded with Ce doped ZnO nanorods, Mater. Res. Bull., 2017, 89, 102–107 CrossRef CAS.
- S. Gawish, H. Mashaly, H. Helmy, A. Ramadan and R. Farouk, Effect of mordant on UV protection and antimicrobial activity of cotton, wool, silk and nylon fabrics dyed with some natural dyes, J. Nanomed. Nanotechnol., 2017, 8, 2 Search PubMed.
- Q. Zhou, J. Lv, L. Cai, Y. Ren, J. Chen, D. Gao, Z. Lu and C. Wang, Preparation and characterization of ZnO/AGE MNPs with aloe gel extract and its application on linen fabric, J. Text. Inst., 2017, 108, 1371–1378 CrossRef CAS.
Footnotes |
| † SSR is used in the laboratory to test the effects of UV irradiation. SSR mimics the spectrum of natural UV radiation under extreme conditions, i.e. a clear cloudless sky at noon at the Equator (zero latitude), but does not contain the visible component of sunlight. SSR spectra vary across different laboratories, which can give rise to different outcomes. |
| ‡ Mendelian randomisation analysis uses genetic variation in an exposure of interest (e.g., 25(OH)D level) to estimate the association between the exposure and a health outcome that is purportedly not due to confounding or reverse causality. |
| This journal is © The Royal Society of Chemistry and Owner Societies 2018 |