 Open Access Article
Open Access ArticleAre current guidelines for sun protection optimal for health? Exploring the evidence†
Robyn M.
Lucas
 *a,
Rachel E.
Neale
*a,
Rachel E.
Neale
 b,
Sasha
Madronich
b,
Sasha
Madronich
 c and
Richard L.
McKenzie
c and
Richard L.
McKenzie
 d
d
aNational Centre for Epidemiology and Population Health, Research School of Population Health, The Australian National University, Canberra, Australia. E-mail: robyn.lucas@anu.edu.au
bQIMR Berghofer Medical Research Institute, Brisbane, Australia
cNational Center for Atmospheric Research, Boulder, Colorado, USA
dNational Institute of Water & Atmospheric Research, NIWA Lauder, Central Otago, New Zealand
First published on 15th June 2018
Abstract
Exposure of the skin to ultraviolet (UV) radiation is the main risk factor for skin cancer, and a major source of vitamin D, in many regions of the world. Sun protection messages to minimize skin cancer risks but avoid vitamin D deficiency are challenging, partly because levels of UV radiation vary by location, season, time of day, and atmospheric conditions. The UV Index provides information on levels of UV radiation and is a cornerstone of sun protection guidelines. Current guidelines from the World Health Organization are that sun protection is required only when the UV Index is 3 or greater. This advice is pragmatic rather than evidence based. The UV Index is a continuous scale; more comprehensive sun protection is required as the UV Index increases. In addition, a wide range of UVA doses is possible with a UVI of 3, from which there may be health consequences, while full sun protection when the UVI is “moderate” (between 3 and 5) may limit vitamin D production. Finally, the duration of time spent in the sun is an essential component of a public health message, in addition to the intensity of ambient UV radiation as measured by the UV Index. Together these provide the dose of UV radiation that is relevant to both skin cancer genesis and vitamin D production. Further education is required to increase the understanding of the UV Index; messages framed using the UV Index need to incorporate the importance of duration of exposure and increasing sun protection with increasing dose of UV radiation.
Introduction
Sun exposure has both harms and benefits. It is the primary risk factor for skin cancer but a major source of vitamin D, in many regions of the world. Providing accurate, evidence-based public health messages about optimal sun exposure is challenging, because ambient ultraviolet (UV) radiation varies according to location, time of day, time of year, and in relation to the weather (e.g. clouds) and other atmospheric conditions, and populations vary with respect to skin types and habitual sun exposure behaviours. Nevertheless, such messages are important globally. In the modern world all skin types are present at all latitudes, and levels of UV radiation exceed the threshold above which WHO guidelines advise sun protection1 in all countries in their summer, and every day of the year for over half of the globe. Peak levels of UV radiation are considered “extreme” for over half of the globe during summer.2Solar UV radiation at Earth's surface is predominantly within the UVA waveband (315–400 nm). UVB wavelengths (280–315 nm) are largely filtered out by stratospheric ozone and make up less than 5% of UV radiation at Earth's surface.3 The intensity of ambient UV radiation decreases with increasing distance from the Equator, is higher in summer than winter, and varies across the day with peak levels at solar noon. This variability relates to the solar zenith angle (SZA) which is the angular distance that the sun is away from being directly overhead. The larger the SZA, the lower the sun is in the sky and the longer the distance through the atmosphere, with more attenuation, particularly of UVB. These factors, along with variability in ozone, alter both the intensity and the spectral composition of UV radiation at Earth's surface, as illustrated in Table 1. This complexity has made it difficult to provide simple messages about how much time people should spend outdoors and when sun protection is needed.
| Conditions | SZA (°) | Total ozone (DU) | UVI | UVB (W m−2) (280–315 nm) | UVA (W m−2) (315–400 nm) |
|---|---|---|---|---|---|
| a Exact value depends on the lower wavelength limit of the integration. Here the lower limit is taken as 280 nm. See Fig. S1 for detailed spectral variations of the irradiances. | |||||
| Extra-terrestrial (altitude 70 km) | 0 | 0 | ∼280a | 14.7 | 85.4 |
| Earth's surface (altitude 0 km) | 0 | 100 | 42.3 | 4.51 | 66.1 |
| 300 | 12.1 | 2.19 | 65.2 | ||
| 450 | 7.4 | 1.47 | 64.6 | ||
| 60 | 100 | 7.9 | 1.19 | 27.3 | |
| 300 | 2.1 | 0.42 | 26.7 | ||
| 450 | 1.4 | 0.23 | 26.0 | ||
The relative biological effectiveness of different wavebands of UV radiation is described by the action spectrum for a specific outcome such as development of erythema (sunburn) or production of vitamin D.4 The action spectra for these two outcomes are different (see Fig. S1†). While UVB wavelengths are most effective for both, UVA wavelengths have some effectiveness for erythema but virtually none for vitamin D production. Action spectra for other outcomes, such as skin cancers, are defined in animal models but not in humans5 and the erythemal action spectrum is most commonly used in relation to adverse effects of UV radiation on human health.
In recent years, the UV Index (UVI), a measure of the erythemal UV radiation at Earth's surface,6 has been used to guide sun protection messages, although public knowledge and understanding of the UVI remains generally poor.7 UVI values are continuous but typically rounded to an integer value and are directly measured in situ or predicted from atmospheric parameters. The UVI is available in weather forecasts, online and through smartphone apps. Messages framed using the UVI obviate the need for specific sun protection messages according to time of day, time of year, and location, as these are already incorporated into the UVI value. Measured UVI also takes account of cloud conditions.
The World Health Organization, through its INTERSUN program, has provided some international consistency in sun protection messaging using the UVI.1 INTERSUN advises that sun protection should be used when the UVI (measured or predicted) is 3 or higher.1 The implicit corollary is that it is safe to be in the sun without protection when the UVI is less than 38 and the guidelines note that at UVI levels of 1 and 2 you can safely stay outside. There is, however, little evidence to support a specific UVI cut-point. In some countries, e.g. Australia, New Zealand and USA, a “UV Sun Protection Alert Period” is issued for the entire period of the day when the forecast clear-sky UVI is 3 or greater (Australia and New Zealand)9 or 6 or greater (USA).10 In some regions in summer, this covers most of the day.
Here we evaluate several issues concerning the use of the UVI to underpin messages about sun protection, integrating published health data with those from atmospheric monitoring of UV radiation. We begin with a brief review of the biological effects of exposure to UV radiation, and the relevant wavebands.
Biological effects of UVB and UVA
Exposure to UV radiation is the primary cause of sunburn, photoageing of the skin, a range of benign lesions such as actinic keratoses, and skin cancers. There appears to be no threshold dose of UV radiation for the induction of skin cancer and thus no safe limit of exposure.11 High-intensity sun exposure also causes photokeratitis and photoconjunctivitis of the eyes, and chronic exposure leads to cataract and pterygium. Both local and systemic immune suppression follow sun exposure and contribute to skin cancer development and the reactivation of latent viral infections (reviewed in ref. 12).The most well-established beneficial effect of skin exposure to UV radiation is production of vitamin D. A number of other potentially beneficial products are formed in the skin following UV irradiation, such as nitric oxide13 and prostaglandin E2,14 but research on these factors is less advanced (reviewed in ref. 12).
Mechanistic studies implicate UVB as the main causative waveband for both skin cancer induction and vitamin D production but there is increasing evidence that the UVA waveband is also important. UVA exposure has been implicated in the development of melanoma and possibly basal cell carcinoma,15,16 as well as in the production of nitric oxide that may have benefits for cardiovascular health.17 Both UVA and UVB irradiation cause immune suppression, acting through a range of different chromophores with specific absorption spectra.18,19 Recent research suggests that UV-induced immune suppression could have both benefits (e.g., suppressing autoimmunity20) and adverse effects (e.g., suppressing immune responses to vaccination21) for human health. Although UVB photons are more effectively immunosuppressant, UV-induced immune suppression may be mainly UVA-induced since there is a peak of immune suppression in the UVA waveband and UVA is much more abundant at Earth's surface than UVB (see Table 1 and Fig. S1†).18
Using the UVI to guide time outdoors and sun protection recommendations
The UVI is a unitless number, defined as 40 times the erythemally weighted UV irradiance, UVEry, expressed in Watts per metre2 (W m−2).| UVI ≡ 40 × UVEry (W m−2) (the definition of UVI) |
Since the UVI is a measure of erythemal UV radiation, messages based on the UVI primarily focus on avoiding sunburn. UVI and UVEry are measures of the intensity of UV radiation at any given moment. However, health effects occur in relation to the total dose of UV radiation, which includes both the intensity and the duration of exposure. The time-averaged erythemal dose (e.g., over 5–10 minutes) is typically provided in units of Joules per m2 (J m−2) or standard erythemal doses (SEDs), where 1 SED = 100 J m−2 of erythemal UV radiation. There is a simple mathematical relationship between the UVI and the number of SEDs as shown below:
The dose received in 1 hour is:
Thus, if UVI = 10, the hourly erythemal dose = 900 J m−2 which is equivalent to 9 SEDs; thus an individual in full sunlight will receive 9 SEDs per hour. A conservative approximation is that the dose of sunburning UV radiation (in SEDs) received in one hour is estimated by the average UVI for that hour. For example, if the average UVI is 6, the number of SEDs received in one hour is ∼6 (actually 5.4, with the approximation providing a small safety factor); thus, 60/UVI approximates the number of minutes to receive 1 SED (on a horizontal unshaded surface).
Individual sensitivity to UV radiation varies, both for erythema and vitamin D production, according to skin type, genetics, age and prior sun exposure (leading to tanning and epidermal hyperplasia).22 For erythema, this is described by the minimal erythema dose (MED) – the dose of erythemally weighted UV radiation that causes just perceptible erythema of the skin. Current measures of skin type, commonly the Fitzpatrick skin-type scale,23 provide a general guide to MED. For fair-skinned Caucasian skin (Fitzpatrick type II), one MED is approximately 2 to 3 SEDs.24 Other examples of these relationships are provided in Zaratti et al. (2014).2
The maximum time that an individual can be outdoors without risking erythema can be estimated from the UVI approximation above and estimates of MED. For example, at a UVI of 6, one hour of exposure delivers ∼6 SED; i.e. 1 SED each 10 min. For a person with skin type II (using a conservative MED of 2 SED), the estimated maximum time outdoors without erythema would be 20 minutes. Of note, the UVI refers to the ambient UV radiation on a horizontal unshaded surface; the calculated exposure thus applies only to a horizontal sunbather, or the vertex of someone standing upright, in an unshaded environment.25 Under other conditions (e.g. in environments where there may be shade from buildings, or on exposed arms and legs while upright) the maximum time required to achieve erythema will generally be longer than this simple calculation, and will vary according to posture, body site exposed and the local environment. This provides additional conservatism in the estimates of time to erythema.
Is avoiding sunburn the appropriate strategy?
Using the UVI to estimate the time to achieve minimal erythema, as outlined above, should help ensure that sunburn does not occur. However, DNA damage occurs even with sub-erythemal doses of UV radiation26 and the damage may persist, at least in an animal model.27 Recent research has shown that human skin contains large numbers of evolving clones of abnormal cells that contain a high proportion of cancer-causing mutations.28 Most of these clones will never develop into a skin cancer; animal studies show that normal (non-mutated) cells in the epidermis actively eliminate mutated cells, replacing the clones with normal skin architecture.29 In addition, a recent laboratory study showed that regular (3 times per week) low dose, non-sunburning (1.3 SED) UV irradiation over 6 weeks was not associated with cumulative DNA damage, as demonstrated by the lack of accumulation of cyclobutane pyrimidine dimers in skin biopsies.30 The accumulation of DNA damage depends on the extent of the damage (a function of the dose of UV radiation and the individual susceptibility) and capacity to eliminate mutated cells or repair DNA (there is some evidence that the latter is impaired by UVA irradiation, at least in laboratory studies).16 Better definition of the kinetics of damage and repair (both of DNA and skin architecture) will inform future modifications of sun protection messages; while the importance of dose to avoid erythema is clear, messages may also need to incorporate recommended non-exposure periods to avoid accumulation of DNA damage.Is UVI less than 3 “safe” without sun protection?
For UVI values commonly encountered at Earth's surface, the development of erythema depends on the dose of UV radiation rather than the rate at which it is delivered (this is termed “reciprocity”).31 Thus, a dose of 2 SED can be received in 20 minutes at a UVI of 6, or in 60 minutes when the UVI is 2, with a similar risk of erythema. This means that, for extended periods outdoors with an average UVI near 3, some protection should clearly be advised. It is inappropriate to base sun protection guidance on UVI reported as a simple binary (e.g. sun protection is required only when the UVI is ≥3), because whether or not protection is required depends on both the UVI and the duration of exposure. Increasing sun protection is required with increasing UVI and with increasing duration of time outdoors.There are several potential health consequences of prolonged exposure to UV radiation at UVI < 3 where, although UVB doses are low, UVA doses may be considerable. First, an erythemal dose can be achieved, as noted above (but with little or no vitamin D production due to the low UVB dose). Second, UVA irradiation has been shown to cause local immune suppression in human studies, with peak effectiveness at 370 nm and with a bell-shaped dose response.18 We note that this is contrary to older evidence from animal studies that exposure to UVA counteracts the effects of UVB-induced immune suppression.32 Whether this reflects the complexity of the effects of UV radiation on immune suppression or a disparity in findings between human and animal studies, is not clear. Third, UVA irradiation causes release of nitric oxide from skin stores, which has beneficial effects on the cardiovascular system.13 Finally, UVA may have at least as much influence as UVB on photoageing.33,34
For any UVI, there is a wide range of possible values of UVA (Fig. 1a). For any UVI value, the largest UVA values occur at larger SZA. In contrast, the relationship between UVI and UVB is approximately linear35 (Fig. 1b).
A UVI value of 3 can be achieved for a wide range of conditions. Table 2 shows examples of ozone amounts (TOZ, total column ozone amount) and cloud transmissions to achieve UVI = 3, and the associated UVA irradiances for selected SZAs.
| SZA (°) | Cloud transmission | TOZ for UVI = 3 (DU) | UVA (W m−2) |
|---|---|---|---|
| 70 | 1.0 | 105 | 16.1 |
| 65 | 1.0 | 160 | 21.2 |
| 60 | 1.0 | 220 | 26.5 |
| 55 | 1.0 | 300 | 31.6 |
| 50 | 1.0 | 380 | 36.5 |
| 45 | 1.0 | 470 | 41.6 |
| 30 | 0.5 | 395 | 27.0 |
| 0 | 0.15 | 200 | 9.9 |
Thus, for clear skies and the normal ranges of ozone (outside the Antarctic ozone hole), UVA can vary by nearly a factor of two for this given UVI value. A UVI value of 3 can also be achieved under cloudy skies at smaller SZAs. Examples are shown in the last two rows of Table 2. If such cloudy conditions are included, UVA can vary by more than a factor of four when the UVI is 3. The actual variability of UVA for a given UVI at any given site depends on the range of ozone and SZA observed at that site, and the cloudiness. For example, at Lauder NZ (45°S) from where the data for Fig. 1 derive, ozone ranges from 200 DU to 450 DU, and the minimum SZA is 22°. At the current UVI threshold for sun protection (UVI = 3, dotted line in Fig. 1a), values of UVA range from 10 to 45 W m−2.
Fig. 2 shows that even in the extreme example where the frequency distributions for UVI > 10 and UVI < 3 are compared, there is occasional overlap in the UVA irradiance.
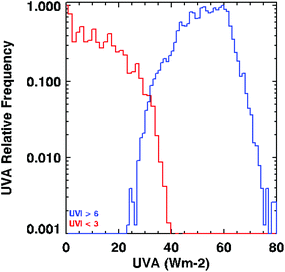 | ||
| Fig. 2 Frequency distribution of UVA (y-axis, normalised to unity in each case) for the case where UVI is less than 3 (red), and for the case where UVI is greater than 10 (blue). The data used are the same as shown in Fig. 1 (i.e., daytime scans only). In nearly 5–10% of cases, the UVA for UVI < 3 is greater than that for UVI > 10. | ||
Given that the UVA irradiance can be relatively high, even at low UVI, we aimed to assess whether plausible levels of UVA irradiance at UVI of 3 or below could have health consequences. While we recognise that animal and human studies provide different findings on the effects of UVA irradiation on immune suppression, local immune suppression is one of the few biological endpoints of UVA irradiation for which there are quantitative data in humans. We use these data to illustrate possible, rather than definitive, health consequences.
Damian and colleagues investigated the relative effects of UVA and UVB irradiation on local immune suppression in 60 nickel-allergic volunteers.36 Our calculations (described in full in ESI, including Tables S1 and S2†) show that with a UVA irradiance of 45 W m−2 (the highest UVA irradiance observed at UVI = 3 in Fig. 1a), maximum immune suppression occurs in 22 minutes (note the uncertainty in the estimate in Fig. S2†).
Although this is an extreme of UVA irradiation that is possible when the UVI is 3, it illustrates that immunosuppression can occur in a relatively short time at UVI just below 3, where current messages advise that no sun protection is required. Fig. 1a shows that when the UVI is 3, the UVA irradiance is typically ∼30 W m−2, which can cause some degree of immune suppression in less than 20 minutes. There is a comparable effect for UVB irradiation, with maximum immune suppression in ∼27 minutes (ESI†).
Two caveats in extrapolating from this study are that immune responses for the general population might be different to those of the allergic people in this study, and that the duration of the immune suppression, and thus its relevance for human health effects, is not clear. Furthermore, the bell-shaped dose–response relationship between UVA and immune suppression may suggest that high doses of UVA could cancel out the suppressive effects of smaller UVA doses.36
Should sun protection always be used when the UVI is 3 or greater?
The best known beneficial effect of sun exposure is vitamin D synthesis. Thus, the achievement and maintenance of vitamin D sufficiency (25(OH)D > 50 nmol L−1) might be used to guide a “healthy/necessary” amount of sun exposure. The currently accepted action spectrum for vitamin D production is shifted slightly toward shorter wavelengths compared to that for erythema (Fig. S1†) and the UVI is a measure of erythemal dose rate. Fig. 1c shows the association between UVI and vitamin D-effective UV radiation. At higher UVI, 1 SED will always include some amount of UVB irradiation (and thus potential for vitamin D synthesis), while at lower UVI, 1 SED may be achieved with mostly UVA irradiation, contributing to erythema but only minimally to vitamin D production.Sun exposure may also have non-vitamin D benefits37 that cannot be achieved with vitamin D supplementation. Observational studies show increased risks of a wide range of diseases in association with 25(OH)D levels <50 nmol L−1.38 However, intervention studies have largely failed to show benefits of vitamin D supplementation for non-skeletal outcomes.39 The exceptions to this are a reduction in all-cause mortality and cancer mortality in middle-aged and older people with supplementation of 10–20 μg day−1,40 and a modest reduction in common upper respiratory tract infections and asthma exacerbations.40 Possible reasons for the discrepancy between observational and intervention studies seen for most health outcomes include reverse causality in observational studies or issues related to trial design such as supplementing people who are not vitamin D deficient, inadequate doses of vitamin D, poor compliance, or supplementing people for too short a time period.41 One other possible explanation for the discrepancy is that it is not vitamin D per se that is beneficial, but rather the sun exposure required to achieve 25(OH)D levels >50 nmol L−1.37 In this case, a serum 25(OH)D level of >50 nmol L−1 could be seen as a marker of “sufficient” sun exposure.
Previous research has shown that 90% of Caucasians (mainly skin type II) achieved vitamin D sufficiency with a dose of UV radiation of 1.3 SEDs (of simulated midday summer sun in Manchester, 53.5°North, UVI ≈ 6) three times weekly for 6 weeks, with 35% of the body surface exposed (short-sleeved shirt and shorts).42 The lowest MED for the study population was 1.6 SED, showing that vitamin D sufficiency occurred without erythema.
Vitamin D sufficiency should be achievable under current messages to use sun protection when the UVI is 3 or greater, provided sun protection is not complete (e.g., there is not complete clothing coverage or sun avoidance such as staying indoors). However, when the UVI is moderate (3 to 5), achieving vitamin D sufficiency may require exposure of unprotected skin for a duration that is not practicable, given environmental (e.g. cool weather that may accompany a moderate UVI) and time constraints, particularly for those with darker skin. For example, achieving vitamin D sufficiency under moderate UVI conditions for darker-skinned individuals may require well over an hour of exposure with 35% of the (horizontal43) body surface exposed. Nevertheless, Farrar and colleagues have shown that exposure of 35% of the body surface area to 1.95 SED (43 min at UVI = 3 or 26 min at UVI = 5) or higher can take darker-skinned individuals out of the vitamin D deficiency range (25(OH)D < 25 nmol L−1).44 If less skin area is exposed, longer exposures will be required45 and the threshold for sunburn to the exposed skin may be exceeded.
Discussion
The UV Index is a convenient tool on which to base messaging around appropriate and safe sun exposure (a summary of considerations is provided in Table S3†). It removes the need to provide messages according to location, season, time of day and atmospheric conditions. It is available in many countries through daily media, online, or through mobile phone apps. Nevertheless, at the moment, understanding of the UVI remains poor in many countries.7,46,47We have shown that with some knowledge of personal skin type, a simple calculation can provide the maximum allowable time outdoors before minimal erythema of the skin occurs. There are safety factors built into this approximation, including that exposure to full sunlight is unlikely for the full duration of the time outdoors, the calculation assumes exposure to a horizontal unshaded surface, and the mathematical approximation itself is conservative. We have also shown that recommendations that sun protection is not required at UVI < 3 may be inappropriate, noting that the duration of time in the sun needs to be considered along with the UVI to avoid sunburn, and local immune suppression may occur even with relatively short exposures. Furthermore, using the full range of sun protection when the UVI is 3 or greater may lead to insufficient sun exposure for vitamin D production.
There have been calls to revise the UVI scale itself to better reflect the extreme values that can occur outside Europe. For example, the maximum category reported is currently 11+, but in some locations the UVI can be more than twice as high as this.2 The use of smartphone apps (e.g., GlobalUV, uv2Day, Uv-indeks) that give information about how the UVI varies throughout the day, along with appropriate messaging, have the potential to facilitate education on the meaning and significance of the UVI that had previously been lacking.
We believe revision of sun protection guidelines based on the UVI is warranted, to avoid the health risks of exposure to UV radiation and to gain the health benefits. Consideration should be given to using the actual UVI rather than categories such as UV Alert periods, and to reporting the UVI according to the hour of the day (rather than just the maximum for the day). A ‘one size fits all’ approach is simple, but may need to be reconsidered to provide appropriate sun protection advice to people with different skin types and/or cultural habits.
The UVI is a continuous scale of UV irradiance – the dose of UV radiation also requires that the duration of exposure, i.e. the time spent in the sun, is incorporated. With increasing UVI, either the duration of exposure needs to decrease, or the sun protection used needs to increase (effectively reducing the dose of UV radiation reaching the skin and/or eyes), or both.
Sun protection messages sometimes consider a hierarchy of protection – from staying indoors when the sun is most intense, through using shade, sunglasses, a hat and covering clothing, to sunscreen. Daily application of sunscreen has been shown to reduce the risk of skin cancer.48 Thus while sunscreen is clearly effective in reducing sunburning when used in an ad hoc way, if skin cancer prevention is the desired endpoint, the evidence-base indicates a message of daily application. A major caveat is that this evidence derives from a very sunny location, and a different message may be more appropriate for other regions of the world.
An additional consideration in public health messaging is the concept of ‘acceptable risk’. Although there is evidence that suberythemal sun exposure interspersed with non-exposed periods does not lead to accumulation of damage30 there is unlikely to be any completely ‘safe’ level of exposure. Thus in order to maintain vitamin D sufficiency, and achieve other possible benefits of some sun exposure, a certain amount of DNA damage – and thus risk of skin cancer – will be incurred.
Conclusions
The UV Index is a valuable tool for health promotion messages around safe sun exposure, but the exposure time is similarly important and must be considered alongside UVI values. Although current public understanding of the UVI is limited, new technologies (e.g., the smartphone apps GlobalUV, uv2Day, Uv-indeks) offer an opportunity to address the issues raised here alongside increased promotion of, and education about, the UVI.49 Effective communication of evidence-based sun exposure/sun protection messages to the public remains a major barrier to achieving significant institutional, collective and individual change in sun exposure behaviours. Current guidelines have been useful but were developed in the absence of specific evidence and are somewhat simplistic. New evidence is now available and should inform revision of guidelines for the future.Glossary
| Astronomical unit: | 1 Astronomical Unit (AU) is the mean separation between Earth and Sun. In Dec/Jan (southern hemisphere summer) the radiation incident outside Earth's atmosphere is about 3.5% more than at 1 AU, and in Jun/Jul (northern hemisphere summer) it is about 3.5% less. |
| Reciprocity of response: | a given degree of the response, e.g. erythema, depends only on the exposure dose of radiation and not on the rate at which the energy is administered. |
| Erythema: | superficial reddening of the skin |
| MED: | minimal erythema dose, the dose of UV radiation that causes minimally perceptible reddening of the skin within a few hours following exposure |
| SED: | standard erythemal dose, equal to 100 J m−2 of erythemally weighted UV radiation |
| UVI: | UV Index, a unitless number that is defined as 40 times the erythemally weighted UV irradiance, when expressed in W m−2 |
| UVA: | UV radiation with wavelength of 315–400 nm |
| UVB: | UV radiation with wavelength from 280–315 nm |
| Action spectrum: | the effectiveness, as a function of specific wavelengths of light, in producing a specific biological endpoint |
Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
Profs Lucas and Neale are funded by Senior Research Fellowships from the National Health and Medical Research Council of Australia.References
- WHO, INTERSUN: The global UV project: a guide and compendium, World Health Organization, 2003 Search PubMed.
- F. Zaratti, R. D. Piacentini, H. A. Guillen, S. H. Cabrera, J. B. Liley and R. L. McKenzie, Proposal for a modification of the UVI risk scale, Photochem. Photobiol. Sci., 2014, 13, 980–985 RSC.
- R. L. McKenzie and S. Madronich, in Encyclopedia of Atmospheric Sciences, ed. J. Holton, J. Pyle and J. Curry, Academic Press, London, 2002, pp. 2474–2480 Search PubMed.
- R. McKenzie, R. Scragg, B. Liley, P. Johnston, J. Wishart, A. Stewart and R. Prematunga, Serum 25-hydroxyvitamin-D responses to multiple UV exposures from solaria: inferences for exposure to sunlight, Photochem. Photobiol. Sci., 2012, 11, 1174–1185 RSC.
- F. R. de Gruijl, Action spectrum for photocarcinogenesis, Recent Results Cancer Res., 1995, 139, 21–30 Search PubMed.
- World Health Organization, Global Solar UV Index, World Health Organization, World Meteorological Organization, United Nations Environment Programme, International Commission on Non-ionizing Radiaton Protection, Geneva, 2002 Search PubMed.
- O. B. Carter and R. J. Donovan, Public (Mis)understanding of the UV Index, J. Health Commun., 2007, 12, 41–52 CrossRef PubMed.
- Cancer Institute NSW, UV Index. The Facts, http://www.darksideoftanning.com.au/pdf/facts-sheet_uv-index.html.
- B. Rajiv and R. Gray, Sun Protection Alert - a simple tool for a complex issue. UV radiation and its effects: an update. Auckland, NZ, 2014 Search PubMed.
- EPA, UV Alert, http://www2.epa.gov/sunwise/uv-alert, (accessed 11 May 2014).
- European Commission Scientific Committee on Health, Opinion on biological effects of ultraviolet radiation relevant to health with particular reference to sunbeds for cosmetic purposes, European Commission, 2016 Search PubMed.
- R. M. Lucas, M. Norval, R. E. Neale, A. R. Young, F. R. de Gruijl, Y. Takizawa and J. C. van der Leun, The consequences for human health of stratospheric ozone depletion in association with other environmental factors, Photochem. Photobiol. Sci., 2015, 14, 53–87 RSC.
- D. Liu, B. O. Fernandez, A. Hamilton, N. N. Lang, J. M. Gallagher, D. E. Newby, M. Feelisch and R. B. Weller, UVA irradiation of human skin vasodilates arterial vasculature and lowers blood pressure independently of nitric oxide synthase, J. Invest. Dermatol., 2014, 134, 1839–1846 CrossRef PubMed.
- N. M. Scott, R. L. Ng, S. Gorman, M. Norval, J. Waithman and P. H. Hart, Prostaglandin E2 imprints a long-lasting effect on dendritic cell progenitors in the bone marrow, J. Leukocyte Biol., 2014, 95, 225–232 CrossRef PubMed.
- F. P. Noonan, M. R. Zaidi, A. Wolnicka-Glubisz, M. R. Anver, J. Bahn, A. Wielgus, J. Cadet, T. Douki, S. Mouret, M. A. Tucker, A. Popratiloff, G. Merlino and E. C. De Fabo, Melanoma induction by ultraviolet A but not ultraviolet B radiation requires melanin pigment, Nat. Commun., 2012, 3, 884 CrossRef PubMed.
- E. Emanuele, J. M. Spencer and M. Braun, From DNA repair to proteome protection: new molecular insights for preventing non-melanoma skin cancers and skin aging, J. Drugs Dermatol., 2014, 13, 274–281 Search PubMed.
- R. B. Weller, Sunlight Has Cardiovascular Benefits Independently of Vitamin D, Blood Purif., 2016, 41, 130–134 CrossRef PubMed.
- D. L. Damian, Y. J. Matthews, T. A. Phan and G. M. Halliday, An action spectrum for ultraviolet radiation-induced immunosuppression in humans, Br. J. Dermatol., 2011, 164, 657–659 Search PubMed.
- G. M. Halliday, S. N. Byrne and D. L. Damian, Ultraviolet A radiation: its role in immunosuppression and carcinogenesis, Semin. Cutaneous Med. Surg., 2011, 30, 214–221 CrossRef PubMed.
- R. M. Lucas, A. L. Ponsonby, K. Dear, P. C. Valery, M. P. Pender, B. V. Taylor, T. J. Kilpatrick, T. Dwyer, A. Coulthard, C. Chapman, I. van der Mei, D. Williams and A. J. McMichael, Sun exposure and vitamin D are independent risk factors for CNS demyelination, Neurology, 2011, 76, 540–548 CrossRef PubMed.
- A. Swaminathan, Doctor of Philosophy, The Australian National University, 2013 Search PubMed.
- F. Xiang, R. Lucas, F. de Gruijl and M. Norval, A systematic review of the influence of skin pigmentation on changes in the concentrations of vitamin D and 25-hydroxyvitamin D in plasma/serum following experimental UV irradiation, Photochem. Photobiol. Sci., 2015, 14, 2138–2146 RSC.
- T. B. Fitzpatrick, The validity and practicality of sun-reactive skin types I through VI, Arch. Dermatol., 1988, 124, 869–871 CrossRef.
- P. Vecchia, M. Hietanen, B. Stuck, E. van Deventer and S. Niu, Protecting workers from ultraviolet radiation, International Commission on non-Ionizing Radiation Protection, 2007 Search PubMed.
- P. Hoeppe, A. Oppenrieder, C. Erianto, P. Koepke, J. Reuder, M. Seefeldner and D. Nowak, Visualization of UV exposure of the human body based on data from a scanning UV-measuring system, Int. J. Biometeorol., 2004, 49, 18–25 CrossRef PubMed.
- S. Seite, A. Fourtanier, D. Moyal and A. R. Young, Photodamage to human skin by suberythemal exposure to solar ultraviolet radiation can be attenuated by sunscreens: a review, Br. J. Dermatol., 2010, 163, 903–914 CrossRef PubMed.
- D. L. Mitchell, B. Volkmer, E. W. Breitbart, M. Byrom, M. G. Lowery and R. Greinert, Identification of a non-dividing subpopulation of mouse and human epidermal cells exhibiting high levels of persistent ultraviolet photodamage, J. Invest. Dermatol., 2001, 117, 590–595 CrossRef PubMed.
- I. Martincorena, A. Roshan, M. Gerstung, P. Ellis, P. Van Loo, S. McLaren, D. C. Wedge, A. Fullam, L. B. Alexandrov, J. M. Tubio, L. Stebbings, A. Menzies, S. Widaa, M. R. Stratton, P. H. Jones and P. J. Campbell, Tumor evolution. High burden and pervasive positive selection of somatic mutations in normal human skin, Science, 2015, 348, 880–886 CrossRef PubMed.
- S. Brown, C. M. Pineda, T. Xin, J. Boucher, K. C. Suozzi, S. Park, C. Matte-Martone, D. G. Gonzalez, J. Rytlewski, S. Beronja and V. Greco, Correction of aberrant growth preserves tissue homeostasis, Nature, 2017, 548, 334–337 CrossRef PubMed.
- S. J. Felton, M. S. Cooke, R. Kift, J. L. Berry, A. R. Webb, P. M. Lam, F. R. de Gruijl, A. Vail and L. E. Rhodes, Concurrent beneficial (vitamin D production) and hazardous (cutaneous DNA damage) impact of repeated low-level summer sunlight exposures, Br. J. Dermatol., 2016, 175, 1320–1328 CrossRef PubMed.
- E. F. Meanwell and B. L. Diffey, Reciprocity of ultraviolet erythema in human skin, Photodermatology, 1989, 6, 146–148 Search PubMed.
- J. Garssen, F. de Gruijl, D. Mol, A. de Klerk, P. Roholl and H. Van Loveren, UVA exposure affects UVB and cis-urocanic acid-induced systemic suppression of immune responses in Listeria monocytogenes-infected Balb/c mice, Photochem. Photobiol., 2001, 73, 432–438 CrossRef PubMed.
- J. H. Rabe, A. J. Mamelak, P. J. McElgunn, W. L. Morison and D. N. Sauder, Photoaging: mechanisms and repair, J. Am. Acad. Dermatol., 2006, 55, 1–19 CrossRef PubMed.
- C. Battie, S. Jitsukawa, F. Bernerd, S. Del Bino, C. Marionnet and M. Verschoore, New insights in photoaging, UVA induced damage and skin types, Exp. Dermatol., 2014, 23(Suppl 1), 7–12 CrossRef PubMed.
- R. McKenzie, D. Smale and M. Kotkamp, Relationship between UVB and erythemally weighted radiation, Photochem. Photobiol. Sci., 2004, 3, 252–256 RSC.
- D. L. Damian, R. S. Barnetson and G. M. Halliday, Low-dose UVA and UVB have different time courses for suppression of contact hypersensitivity to a recall antigen in humans, J. Invest. Dermatol., 1999, 112, 939–944 CrossRef PubMed.
- P. H. Hart, S. Gorman and J. J. Finlay-Jones, Modulation of the immune system by UV radiation: more than just the effects of vitamin D?, Nat. Rev. Immunol., 2011, 11, 584–596 CrossRef PubMed.
- M. Peterlik, Vitamin D insufficiency and chronic diseases: hype and reality, Food Funct., 2012, 3, 784–794 RSC.
- M. J. Bolland, A. Grey, G. D. Gamble and I. R. Reid, The effect of vitamin D supplementation on skeletal, vascular, or cancer outcomes: a trial sequential meta-analysis, Lancet Diabetes Endocrinol., 2014, 2, 307–320 CrossRef PubMed.
- P. Autier, P. Mullie, A. Macacu, M. Dragomir, M. Boniol, K. Coppens, C. Pizot and M. Boniol, The influence of vitamin D supplementation on non-skeletal conditions: a systematic review of randomised trials and of their meta-analyses, Lancet Diabetes Endocrinol., 2017, 5, 986–1004 CrossRef PubMed.
- J. M. Lappe and R. P. Heaney, Why randomized controlled trials of calcium and vitamin D sometimes fail, Dermatoendocrinol., 2012, 4, 95–100 CrossRef PubMed.
- L. E. Rhodes, A. R. Webb, H. I. Fraser, R. Kift, M. T. Durkin, D. Allan, S. J. O'Brien, A. Vail and J. L. Berry, Recommended summer sunlight exposure levels can produce sufficient (> or = 20 ng ml(-1)) but not the proposed optimal (> or = 32 ng ml(-1)) 25(OH)D levels at UK latitudes, J. Invest. Dermatol., 2010, 130, 1411–1418 CrossRef PubMed.
- M. Schrempf, N. Thuns, K. Lange and G. Seckmeyer, Impact of Orientation on the Vitamin D Weighted Exposure of a Human in an Urban Environment, Int. J. Environ. Res. Public Health, 2017, 14, E920 CrossRef PubMed.
- M. D. Farrar, A. R. Webb, R. Kift, M. T. Durkin, D. Allan, A. Herbert, J. L. Berry and L. E. Rhodes, Efficacy of a dose range of simulated sunlight exposures in raising vitamin D status in South Asian adults: implications for targeted guidance on sun exposure, Am. J. Clin. Nutr., 2013, 97, 1210–1216 CrossRef PubMed.
- M. K. Bogh, A. V. Schmedes, P. A. Philipsen, E. Thieden and H. C. Wulf, Interdependence between body surface area and ultraviolet B dose in vitamin D production: a randomized controlled trial, Br. J. Dermatol., 2011, 164, 163–169 CrossRef PubMed.
- J. Morris, T. Laing-Morton, P. Marno and A. Curnow, An investigation into the awareness and understanding of the ultraviolet index forecasts in the South West of England, Photochem. Photobiol. Sci., 2011, 10, 103–108 RSC.
- C. Y. Wright, A. I. Reeder and P. N. Albers, School students’ knowledge and understanding of the Global Solar Ultraviolet Index, S. Afr. Med. J., 2015, 105, 1024–1029 CrossRef PubMed.
- A. C. Green, G. M. Williams, V. Logan and G. M. Strutton, Reduced melanoma after regular sunscreen use: randomized trial follow-up, J. Clin. Oncol., 2011, 29, 257–263 CrossRef PubMed.
- L. Finch, M. Janda, L. J. Loescher and E. Hacker, Can skin cancer prevention be improved through mobile technology interventions? A systematic review, Prev. Med., 2016, 90, 121–132 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: All calculations and supporting text, Tables S1–S3 and Fig. S1 and S2. See DOI: 10.1039/c7pp00374a |
| This journal is © The Royal Society of Chemistry and Owner Societies 2018 |

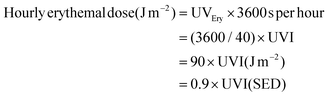
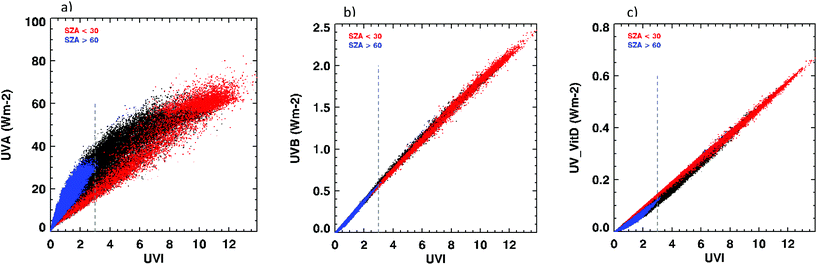
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 spectral scans taken over the period 2001 to 2015 covering a range of SZA from 22° to 90°. Scans for SZA < 30° (high sun) are highlighted in red, while scans for SZA > 60° (low sun) are highlighted in blue. Effects of temporal changes (
000 spectral scans taken over the period 2001 to 2015 covering a range of SZA from 22° to 90°. Scans for SZA < 30° (high sun) are highlighted in red, while scans for SZA > 60° (low sun) are highlighted in blue. Effects of temporal changes (