Metal-free radical cascade chloromethylation of unactivated alkenes: synthesis of polychloro-substituted indolines†
Abstract
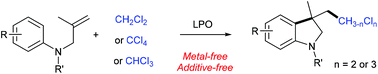
The di- and trichloromethylation of N-allyl anilines was developed under metal-free conditions, leading to a variety of di- and trichloromethylated indolines in moderate to good yields. This procedure used the commercially available chemical feedstocks CH2Cl2, CHCl3 and CCl4 as the di- and trichloromethyl sources.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...