Enantioselective total synthesis of decytospolide A and decytospolide B using an Achmatowicz reaction†
Abstract
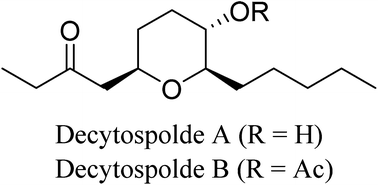
Enantioselective syntheses of decytospolide A and decytospolide B are described here. The current synthesis highlights an Achmatowicz rearrangement of an optically active furanyl alcohol followed by reduction of the resulting dihydropyranone hemiacetal with BF3·OEt2 and Et3SiH to provide the saturated tetrahydropyranyl alcohol directly. This reduction was investigated with a variety of other Lewis acids. The synthesis also features Noyori asymmetric transfer hydrogenation and Friedel–Crafts acylation. Overall, the synthesis provides ready access to the natural products and may be useful in the preparation of bioactive derivatives.
- This article is part of the themed collection: Total synthesis in OBC


 Please wait while we load your content...
Please wait while we load your content...