Metal-free C(sp3)–H bond sulfonyloxylation of 2-alkylpyridines and alkylnitrones†
Abstract
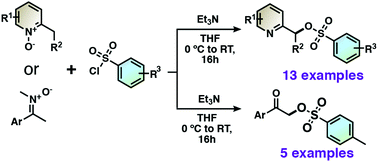
Pyridin-2-ylmethyl tosylate derivatives are obtained in high yields from 2-alkylpyridine 1-oxides via a [3,3]-sigmatropic rearrangement of the adduct between 2-alkylpridine 1-oxides with benzenesulfonyl chlorides. Moreover, alkylnitrones also undergo [3,3]-sigmatropic rearrangement to give α-tosylated ketones after hydrolysis. Substitution reactions with nucleophiles then lead to diverse useful functionalizations for the synthesis of pincer ligands.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...