Electrochemical Hofmann rearrangement mediated by NaBr: practical access to bioactive carbamates†
Abstract
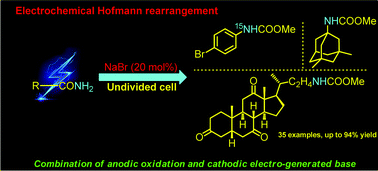
An electrochemical Hofmann rearrangement is reported. With the mediation of NaBr, highly corrosive and toxic halogens are avoided. Moreover, this efficient and green approach is well compatible with a broad range of amides, including several commercial medicine derivatives, and provides direct access to synthetically useful carbamates. The synthetic utility of this method is also demonstrated by the preparation of 15N labeling carbamate and gram-scale synthesis of Amantadine.
- This article is part of the themed collection: Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...