Aziridine based electrophilic handle for aspartic acid ligation†
Abstract
A one-pot ligation strategy at aspartic acid junctions has been developed by successfully incorporating aziridin-2,3-dicarboxylate to the N-side of a peptide fragment, affording N-aziridine appended peptides, which were ligated in solution phase with a variety of small peptide thio acids to afford native peptides, following a ring-opening/peptidyl migration/desulfurization strategy. The reaction proceeds in a highly regiospecific manner, and provides short native peptides in good isolable yields. A variety of aspartame based peptides were synthesized to showcase the generality of this aziridine based ligation. Computational studies have also been performed to obtain insight about the reaction pathway.
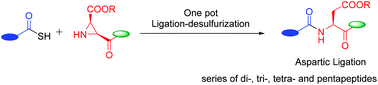
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...