Site-specific hydroxyalkylation of chromones via alcohol mediated Minisci-type radical conjugate addition†
Abstract
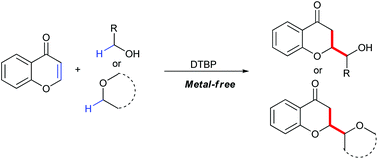
A metal-free site-specific C2-hydroxyalkylation of chromones through the Minisci-type reaction using simple alcohols was developed. This transformation proceeds via radical sp3 C–H activation and subsequent conjugate addition, generating a series of C2-hydroxyalkylated chromanones in moderate to good yields. Besides, ethers were also compatible in this Minisci reaction, leading to corresponding C2 ether-substituted chromanones.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...