Synthetic studies toward the marine metabolite prorocentin-4: synthesis of the C1–C23 fragment†
Abstract
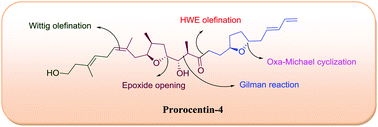
A synthetic study of the construction of the C1–C23 fragment of prorocentin-4, a novel linear polyketide, is described. The synthetic highlights include the acid catalyzed epoxide opening, Gilman reaction, Pd(OH)2 catalyzed transformation of a primary propargylic alcohol into an aldehyde, Oxa-Michael cyclization, and Horner–Wadsworth–Emmons (HWE) olefination reaction as key steps.
- This article is part of the themed collection: Total synthesis in OBC


 Please wait while we load your content...
Please wait while we load your content...