In vivo and in vitro identification of Z-BOX C – a new bilirubin oxidation end product†
Abstract
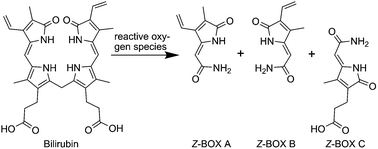
A new bilirubin oxidation end product (BOX) was isolated and characterized. The formation of the so-called Z-BOX C proceeds from bilirubin via propentdyopents as intermediates. This BOX was detected in pathological human bile samples using liquid chromatography/mass spectrometry and has potential relevance for liver dysfunction and cerebral vasospasms.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...