Synthesis and characterization of water-soluble macrocyclic peptides stabilizing protein α-turn†
Abstract
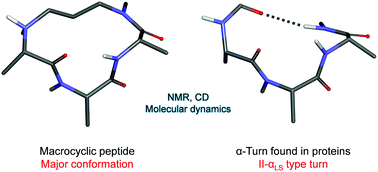
Short peptides composed of naturally occurring amino acids are usually unstructured in aqueous media. The installation of covalent constraints within their side chains or backbones, resulting in the formation of macrocyclic peptides, is an appealing approach to stabilize them in defined secondary structures. Therefore, with the objective to stabilize α-turn conformation, we designed, synthesized and characterized constrained 13-membered macrocyclic peptides. Their design was inspired by previous work using the replacement of a hydrogen bond by a covalent bond, for the stabilization of α-helical secondary structures. Their synthesis employed our recently published solid-phase method based on Fukuyama–Mitsunobu alkylation reactions. We report herein an optimized synthesis leading to three water-soluble 13-membered macrocyclic peptides 10a–c, including respectively two, one and zero glycine residues. They were characterized by CD and NMR, which indicated the presence of equilibrating conformers. The detailed conformational analysis was based on extensive NMR and molecular dynamics studies. We found that the peptide without glycine residues 10c was mostly present as slowly interconverting conformers whereas the peptide with two glycine residues 10a was mostly present as rapidly interconverting conformers. We did not find a good match between the conformers of 10a and α-turns occurring in proteins, due to the high flexibility of the glycine backbone. Interestingly, we found that the major conformer of 10c accurately matched the “non-classical” or “tight” α-turn of type II-αLS, with a RMSD value of 0.42 Å for heavy atoms constituting the macrocycle. This is, to the best of our knowledge, the first molecule reported to mimic this type of α-turn found in proteins.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...