Convenient synthesis of 2-amino-3-(arylthio)indoles via the Rh-catalyzed reaction of 3-diazoindol-2-imines with thioesters†
Abstract
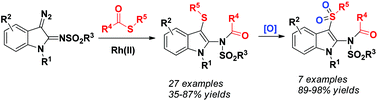
2-Amino-3-(arylthio)indoles were conveniently synthesized via the Rh(II)-catalyzed C–S/N–C coupling reaction between 3-diazoindol-2-imines and thioesters. The products could be further oxidized to 2-amino-3-(arylsulfonyl)indoles by m-chloroperbenzoic acid and the N-sulfonyl group could be easily removed by reduction with SmI2.


 Please wait while we load your content...
Please wait while we load your content...