Inherent electrochemistry and charge transfer properties of few-layered two-dimensional Ti3C2Tx MXene†
Abstract
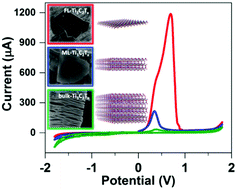
We report the effect of the Ti3C2Tx MXene flake thickness on its inherent electrochemistry and heterogeneous charge transfer characteristics. It is shown that Ti3C2Tx undergoes irreversible oxidation in a positive potential window, which strongly depends on the flake thickness and pH of the electrolyte. Few-layered Ti3C2Tx exhibits faster electron transfer kinetics (k0 = 0.09533 cm s−1) with a [Fe(CN)6]4−/3− redox mediator compared to multi-layered Ti3C2Tx (k0 = 0.00503 cm s−1). In addition, the few-layered free standing Ti3C2Tx film electrode remains intact after enduring irreversible oxidation.


 Please wait while we load your content...
Please wait while we load your content...