Ultrahigh photosensitivity of the polar surfaces of single crystalline ZnO nanoplates
Abstract
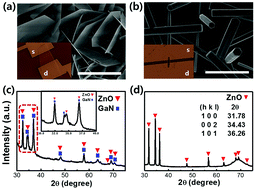
Single crystalline ZnO nanoplatelet structures were synthesized via a hydrothermal process on the surface of GaN microparticles. Growth of ZnO seeded on the GaN surface promoted faster growth along the directions within the basal plane of the ZnO crystal structure, resulting in the formation of 2-dimensional nanoplates with a thickness less than a few tens of nanometers at most. Electrical conduction across an individual nanoplate was measured and found to be extremely sensitive to UV illumination and the surrounding atmospheric environment. Such electrical behaviors of the nanoplates were attributed to the dominance of the polar (0001) surfaces and the adsorption and desorption of the ambient gas molecules on these surfaces. Their coupling with conduction electrons near the surface is the critical factor responsible for the highly sensitive electrical properties of the nanoplate. Virtually the entire volume of the nanoplates is under the influence of the surface adsorbed molecules, which changes the electrical properties of the nanoplates extensively, depending on their environmental conditions. Combining the very high photocurrent to dark current ratio and the high effective resistance of the ZnO nanoplates reported in the present study, ultrasensitive photo-devices operating at very low power can be expected with the use of 2-dimensional nanoplates.


 Please wait while we load your content...
Please wait while we load your content...