A novel preparation method for ZnO/γ-Al2O3 nanofibers with enhanced absorbability and improved photocatalytic water-treatment performance by Ag nanoparticles†
Abstract
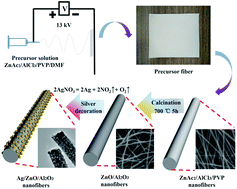
A novel method for synthesizing ZnO/γ-Al2O3 nanofibers by electrospinning and subsequent calcination is reported. The prepared nanofibers were characterized by scanning electron microscopy (SEM), transmission electron microscopy (TEM), energy-dispersive X-ray spectroscopy (EDS), X-ray diffraction (XRD), and X-ray photoelectron spectroscopy (XPS). The ZnO/γ-Al2O3 nanofibers exhibited excellent capacity for adsorbing organics with a negative zeta potential such as methyl orange (95.8%) and heavy metal ions such as Cr(VI) in aqueous solution. The mechanism of adsorption was investigated, and the adsorption results were fitted using the Langmuir and Freundlich models. Once silver nanoparticles (Ag NPs) were decorated on the surface of the nanofibers by photoreduction, the Ag/ZnO/γ-Al2O3 nanofibers manifested efficient photocatalytic degradation of methyl orange under UV-light illumination. Results confirmed that our Ag/ZnO/γ-Al2O3 nanofibers are a promising adsorbent for the removal of methyl orange and Cr(VI) ions and the adsorbent can be sustainably reused.


 Please wait while we load your content...
Please wait while we load your content...