Surface ligand modification of cesium lead bromide nanocrystals for improved light-emitting performance†
Abstract
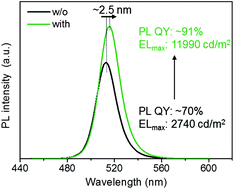
Cesium lead halide perovskite nanocrystals (NCs) possess excellent optical properties at visible wavelengths with great promise for applications in luminous display fields. We demonstrate a method to modify the surface ligand passivation of perovskite NCs for enhanced colloidal stability and emitting properties by incorporating didodecyl dimethyl ammonium bromide (DDAB). The photoluminescence quantum yield of the NC solution was improved to 96% from 70% and the perovskite film showed fewer trapped sites and enhanced carrier transport ability. The thus fabricated electroluminescent perovskite NC-LEDs exhibited a bright luminance of 11 990 cd m−2, corresponding to 4-times improved external quantum efficiency (EQE), compared to the control device using regular NCs without DDAB.
- This article is part of the themed collection: Editor’s Choice: Perovskite Nanomaterials and Devices


 Please wait while we load your content...
Please wait while we load your content...