Role of topological scale in the differential fouling of Pseudomonas aeruginosa and Staphylococcus aureus bacterial cells on wrinkled gold-coated polystyrene surfaces†
Abstract
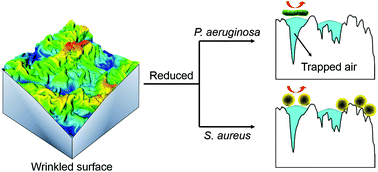
Wrinkled patterns, which possess an extensive surface area over a limited planar space, can provide surface features ranging across the nano- and microscale that have become an engineering material with the flexibility to be tuneable for a number of technologies. Here, we investigate the surface parameters that influence the attachment response of two model bacteria (P. aeruginosa and S. aureus) to wrinkled gold-coated polystyrene surfaces having topologies at the nano- and microscale. Together with flat gold films as the controls, surface feature heights spanned 2 orders of magnitude (15 nm, 200 nm, and 1 micron). The surface wrinkle topology was shown through confocal laser scanning microscopic, atomic force microscopic and scanning electron microscopic image analyses to consist of air–water interfacial areas unavailable for bacterial attachment, which were also shown to be stable by time-lapsed contact angle measurements. Imposition of the nanoscale wrinkles reduced P. aeruginosa attachment to 57% and S. aureus attachment to 20% of their flat equivalent surfaces whereas wrinkles at the microscale further reduced these attachments to 7.5% and 14.5%, respectively. The density of attachments indicated an inherent species specific selectivity that changed with feature dimension, attributable to the scale of the air–water interfaces in contact with the bacterial cell. Parameters influencing static bacterial attachment were the total projected surface areas minus the air–water interface areas and the scale of these respective air–water interfaces (area distribution) with respect to the cell morphology. The range of these controlling parameters may provide new design principles for the evolving suite of physical anti-biofouling materials not reliant on biocidal agents under development.


 Please wait while we load your content...
Please wait while we load your content...