An open holey structure enhanced rate capability in a NaTi2(PO4)3/C nanocomposite and provided ultralong-life sodium-ion storage†
Abstract
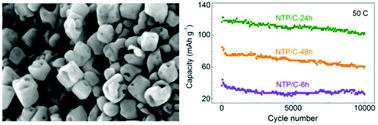
Sodium-ion battery (SIB) technology is competitive in the fields of transportation and grid storage, which require electrode materials showing rapid energy conversion (high rate capability) and long cycle life. In this work, a NaTi2(PO4)3/C (NTP/C) nanocomposite with an open holey-structured framework was successfully prepared for the first time using a solvothermal reaction followed by pyrolysis. The nanocomposite realized fast sodium-ion transport and thus preferable battery performances. Within the wide rate range of 0.5–50C, only a very small decrease in capacity from 124 to 120 mA h g−1 was observed. A high discharge capacity of 103 mA h g−1 (88.3% retention of the 1st cycle) was delivered even after 10 000 cycles at an ultrahigh rate of 50C without any obvious morphological change and without structural pulverization. Forming open channels for ion transport proved to contribute to such performance enhancement and therefore has the potential to become a universal model for the development of sustainable electrode materials in SIBs and other battery systems.


 Please wait while we load your content...
Please wait while we load your content...