Surface engineering for improved stability of CH3NH3PbBr3 perovskite nanocrystals†
Abstract
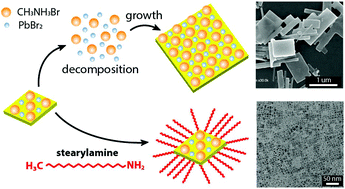
Organohalide perovskite nanocrystals (NCs) with a variety of nano-scale structures and morphologies have shown promising potential owing to their size- and composition-dependent optoelectronic properties. Despite extensive studies on their size-dependent optical properties, a lack of understanding on their morphological transformation and the relevant stability issues limits a wide range of applications. Herein, we hypothesize a mechanism for the morphological transformation of perovskite NCs, which leads to dissolving NCs and forming microscale rectangular grains, resulting in a reduction of photoluminescence. We found that the morphological transformation from nanocrystal solids to microscale rectangular solids occurs via Ostwald ripening. A surface treatment with a surfactant suppresses the transformation, resulting in nearly monodisperse NCs with a square shape (∼20 nm edge size), and thus improves the stability of NC solution, as well as their photoluminescence performance and quantum yield (PLQY = 82%). Furthermore, we employed similar amine derivatives to investigate the effect of a molecular architecture (i.e. steric hindrance) on perovskite NC stability, which exhibited much enhanced PLQY (93%). These experimental results provide new insights into the fundamental relationship between the physical properties and the structure of perovskite nanocrystals required to understand their diverse optoelectronic properties.
- This article is part of the themed collection: Editor’s Choice: Perovskite Nanomaterials and Devices


 Please wait while we load your content...
Please wait while we load your content...